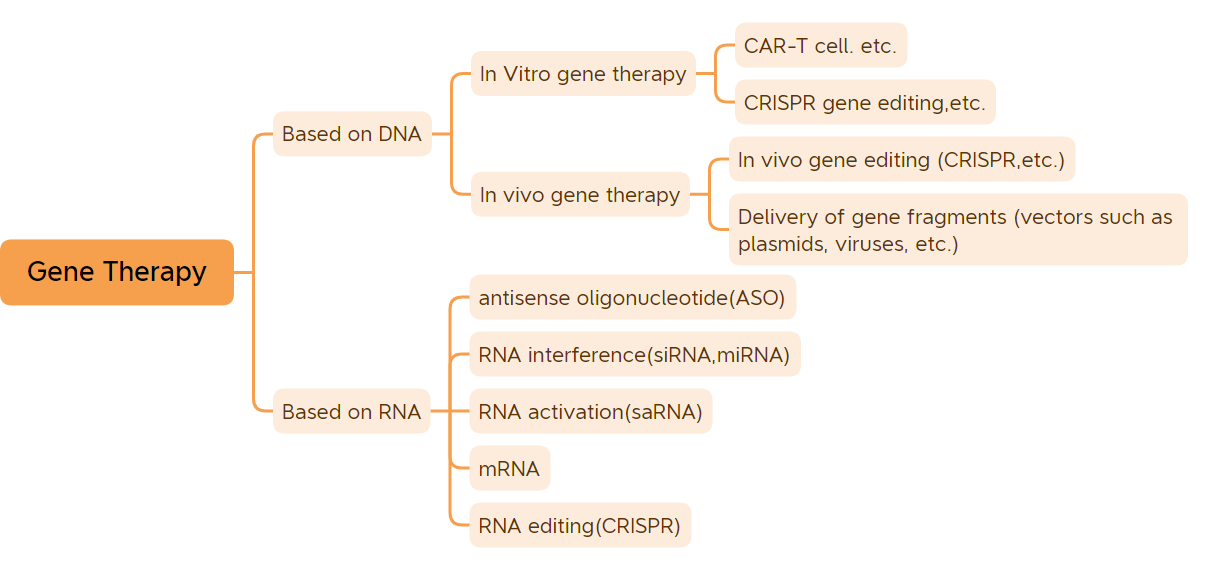
Imfuza ziiyunithi ezisisiseko zofuzo ezilawula iimpawu.Ngaphandle kwemizila yemfuza yezinye iintsholongwane, eziyilwe yiRNA, imizila yemfuza yezinto ezininzi iyilwe yiDNA..Uninzi lwezifo zezinto eziphilayo zibangelwa yintsebenziswano phakathi kwemfuza kunye nokusingqongileyo.Unyango lwemfuza lunokunyanga okanye luthomalalise izifo ezininzi.Unyango lweGene luthathwa njengenguquko kwicandelo lamayeza kunye nekhemesti.Iziyobisi zonyango lwe-Gene ngengqiqo ebanzi zibandakanya ngokusekelwe kumachiza e-DNA-modified DNA (ezifana namachiza e-vivo gene therapy esekelwe kwi-viral vectors, i-in vitro gene therapy amayeza, amachiza e-plasmid ehamba ze, njl.Ingqiqo emxinwa amachiza onyango lwe-Gene ikakhulu abandakanya amachiza e-plasmid DNA, amachiza onyango lwemfuza asekwe kwiintsholongwane zentsholongwane, amayeza onyango lwemfuza asekelwe kwiintsholongwane zebhaktiriya, iinkqubo zokuhlela imizila yemfuza, kunye namachiza onyango lweeseli zokuguqulwa kofuzo kwi-vitro.Emva kweminyaka yophuhliso olubi, amachiza onyango lwemfuza afumene iziphumo ezikhuthazayo zeklinikhi.(Kungabalwa izitofu ze-DNA kunye nogonyo lwe-mRNA) Okwangoku, amachiza onyango lwemfuza angama-45 avunyiweyo ukuba athengiswe kwihlabathi.Iyonke i-9 yonyango yofuzo ivunyiwe ukuba ithengiswe kulo nyaka, kubandakanywa amayeza esi-7 avunyiweyo ukuthengiswa okokuqala kulo nyaka, angala: CARVYKTI, Amvuttra, Upstaza, Roctavian, Hemgenix, Adstiladrin kunye ne-Ebvallo, (Qaphela: Ezinye ezimbini zamkelwe eUnited States kulo nyaka. I-FDA yokuthengisa e-United States ngo-Agasti ka-2022, kwaye yamkelwa ukuba ithengiswe yiManyano yaseYurophu ngo-2019;.) Ngokusungulwa kweemveliso ezininzi zonyango lwemfuza kunye nophuhliso olukhawulezayo lweteknoloji yonyango lwemfuza, unyango lwemfuza sele luza kungenisa ixesha lophuhliso olukhawulezayo.
Ulwahlulo lonyango lwemfuza (Umthombo womfanekiso: Bio-Matrix)
Eli nqaku lidwelisa i-45 yemfuza yonyango (ngaphandle kogonyo lwe-DNA kunye nogonyo lwe-mRNA) oluvunyiweyo ukuba luthengiswe.
1. Unyango lwemfuza ye-in vitro
(1) Strimvelis
Inkampani: Iphuhliswe yiGlaxoSmithKline (GSK).
Ixesha lokuthengisa: Yavunywa ukuba ithengiswe yiManyano yaseYurophu ngoMeyi ka-2016.
Izibonakaliso: Kunyango lwe-immunodeficiency edibeneyo (SCID).
Amanqaku: Inkqubo eqhelekileyo yolu nyango kukuqala ukufumana iiseli ze-hematopoietic stem zesigulane, ukwandisa kunye nenkcubeko kubo kwi-vitro, emva koko usebenzise i-retrovirus ukwazisa i-ADA esebenzayo (i-adenosine deaminase) ikopi yofuzo kwiiseli ze-hematopoietic stem, kwaye ekugqibeleni ifake iiseli ze-Hematopoietic stem eziguqulwayo zibuyiselwa emzimbeni.Iziphumo zezonyango zibonisa ukuba izinga lokusinda leminyaka emi-3 yezigulane ze-ADA-SCID ezinyangwa nge-Strimvelis yi-100%.
(2) I-Zalmoxis
Inkampani: Iveliswe yiNkampani yeMolMed yaseItali.
Ixesha lokuthengisa: Ifunyenwe isigunyaziso sokuthengisa esinemiqathango kwi-European Union ngo-2016.
Iimpawu: Isetyenziselwa unyango lwe-adjuvant ye-immune system yezigulane emva kokutshintshwa kwe-hematopoietic stem cell.
Amagqabantshintshi: I-Zalmoxis yi-allogeneic T cell suicide gene immunotherapy ehlengahlengisiweyo zii-retroviral vectors.Le ndlela isebenzisa ii-retroviral vectors to genetically modify allogeneic cell cells, ukwenzela ukuba iiseli ze-T eziguqulwe ngokwemfuza ziveze i-1NGFR kunye ne-HSV-TK Mut2 yokuzibulala izakhi zofuzo zivumela abantu ukuba basebenzise iziyobisi ze-ganciclovir (ganciclovir) nangaliphi na ixesha ukubulala iiseli ze-T ezibangela ukusabela okungahambi kakuhle komzimba, ukuthintela ukuwohloka okuqhubekayo kwe-immune ye-GVHD kunye nokubonelela nge-postoperative haploden.
(3) Invossa-K
Inkampani: Iphuhliswe yiTissueGene (KolonTissueGene).
Ixesha lokuthengisa: Ivunyiwe ukuba ifakwe kuluhlu eMzantsi Korea ngoJulayi ka-2017.
Izibonakaliso: Kunyango lwe-arthrosis yamadolo.
Amagqabantshintshi: I-Invossa-K lunyango lwe-allogeneic cell gene olubandakanya iichondrocyte zabantu.Iiseli ze-allogeneic ziguqulwa ngokwe-genetically modified in vitro, kwaye iiseli eziguquliweyo zinokuvakalisa kwaye zikhuphele ukuguqula ukukhula kwe-β1 (TGF-β1) emva kwe-injection ye-intra-articular.β1), ngaloo ndlela kuphuculwe iimpawu ze-osteoarthritis.Iziphumo zeklinikhi zibonisa ukuba i-Invossa-K inokuphucula kakhulu i-arthritis yamadolo.Yarhoxiswa ngo-2019 nguLawulo lokuTya kunye neziyobisi lwaseKorea kuba umenzi wazibhala kakubi izithako ezisetyenzisiweyo.
(4) Zynteglo
Inkampani: Iphandwe yaphuhliswa yibluebird bio.
Ixesha lokuthengisa: Ivunywe yiManyano yaseYurophu ukuba ithengiswe ngo-2019, kwaye yamkelwe yi-FDA ukuze ithengiswe eUnited States ngo-Agasti ka-2022.
Iimpawu: Kunyango lwe-β-thalassemia exhomekeke kutofelo-gazi.
Amanqaku: I-Zynteglo yi-lentiviral in vitro gene gene therapy eyenza ikopi esebenzayo ye-beta-globin gene yesiqhelo (βA-T87Q-globin gene) kwiiseli ze-hematopoietic stem ezithathwe kwisigulane ngokusebenzisa i-lentival vector, kwaye iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iphinde iguqule i-autologous stem cell cell.Emva kokuba isigulane sine-gene ye-βA-T87Q-globin eqhelekileyo, inokuvelisa iprotheyini ye-HbAT87Q eqhelekileyo, enokunciphisa ngokufanelekileyo okanye yokuphelisa isidingo sokutofelwa igazi.Lunyango lwexesha elinye olwenzelwe ukutshintsha utofelo-gazi lobomi kunye namayeza obomi bonke kwizigulana ezineminyaka eli-12 ubudala nangaphezulu.
(5) Skysona
Inkampani: Iphandwe yaphuhliswa yibluebird bio.
Ixesha lokuthengisa: Ivunywe yiManyano yaseYurophu ukuba ithengiswe ngoJulayi 2021, kwaye yamkelwe yi-FDA ukuze ithengiswe eMelika ngoSeptemba ka-2022.
Izibonakaliso: Kunyango lwe-cerebral adrenoleukodystrophy (CALD) yokuqala.
Amagqabantshintshi: Unyango lofuzo lwe-Skysona lolona nyango lofuzo lwexesha elinye oluvunyiweyo kunyango lwe-cerebral adrenoleukodystrophy (CALD) lwakwangoko.I-Skysona (elivaldogene autotemcel, Lenti-D) yi-hematopoietic stem cell lentiviral in vitro gene therapy therapy Lenti-D.Inkqubo eqhelekileyo yonyango ngolu hlobo lulandelayo: iiseli ze-autologous hematopoietic stem zithathwa kwisigulane, zidluliselwe kwaye zitshintshwe yi-lentivirus ephethe i-ABCD1 gene in vitro, kwaye iphinde ibuyiselwe kwisigulane.Isetyenziselwa ukuphatha izigulane ezingaphantsi kweminyaka eyi-18, ithwala i-ABCD1 yokuguqulwa kofuzo, kunye ne-CALD.
(6) Kymriya
INKAMPANI: Iphuhliswe nguNovartis.
Ixesha lokuthengisa: Ivunywe yi-FDA yokuthengisa ngo-Agasti 2017.
Izibonakaliso: Unyango lwe-precursor B-cell acute lymphoblastic leukemia (ALL) kunye ne-DLBCL ephindaphindiweyo kunye ne-refractory.
Amanqaku: I-Kymriah lichiza lonyango lwe-lentiviral in vitro gene, unyango lokuqala lwe-CAR-T oluvunyiweyo ukuthengiswa kwihlabathi, lujolise kwi-CD19, kunye nokusetyenziswa kwe-4-1BB co-stimulatory factor.Ixabisa i-$475,000 e-US kunye ne-$313,000 eJapan.
(7) Yescarta
Inkampani: Iphuhliswe nguKite Pharma, isebe leGiliyadi (GILD).
Ixesha lokuthengisa: Ivunywe yi-FDA yokuthengisa ngo-Oktobha 2017;I-Fosun Kite yazisa itekhnoloji ye-Yescarta evela kwi-Kite Pharma kwaye yavelisa e-China emva kokufumana isigunyaziso.Ivunyiwe ukuba idweliswe elizweni.
Izalathiso: Kunyango lwe-lymphoma enkulu ebuyileyo okanye echasayo.
Amanqakwana: I-Yescarta lunyango lwe-retroviral in vitro gene therapy, lunyango lwe-CAR-T lwesibini oluvunyiweyo kwihlabathi.Ijolise kwi-CD19 kwaye yamkele i-costimulator ye-CD28.Ixabisa i-373,000 yeedola eUnited States.
(8) ITecartus
Inkampani: Iphuhliswe yiGiliyadi (GILD).
Ixesha lokuthengisa: Ivunywe yi-FDA ukuze ithengiswe ngoJulayi ka-2020.
Izalathisi: Kwi-mantle cell lymphoma ebuyele umva okanye irefractory.
Amagqabantshintshi: I-Tecartus lunyango lwe-CAR-T lwe-autologous olujolise kwi-CD19, kwaye lunyango lwesithathu lwe-CAR-T oluvunyiweyo ukuthengiswa kwihlabathi.
(9) Breyanzi
INKAMPANI: Iphuhliswe yiBristol-Myers Squibb (BMS).
Ixesha lokuthengisa: Ivunywe yi-FDA yokuthengisa ngoFebruwari 2021.
Izibonakaliso: I-Relapsed or refractory (R / R) enkulu ye-B-cell lymphoma (LBCL).
Amanqaku: I-Breyanzi yi-in vitro gene therapy esekelwe kwi-lentivirus, unyango lwesine lwe-CAR-T oluvunyiweyo ukuthengiswa kwihlabathi, lujolise kwi-CD19.Ukuvunywa kweBreyanzi sisiganeko esibalulekileyo seBristol-Myers Squibb kwinkalo ye-cellular immunotherapy, eyayifumana xa ifumana iCelgene nge-74 yeebhiliyoni zeedola ngo-2019.
(10) Abecma
Inkampani: Iphuhliswe yiBristol-Myers Squibb (BMS) kunye ne-bluebird bio.
Ixesha lokuthengisa: Ivunywe yi-FDA ukuze ithengiswe ngoMatshi ka-2021.
Iimpawu: i-myeloma ephindaphindiweyo okanye i-refractory.
Amagqabantshintshi: I-Abecma lunyango lofuzo lwe-in vitro olusekwe kwi-lentivirus, unyango lokuqala lwehlabathi lwe-CAR-T lweseli olujolise kwi-BCMA, kunye nonyango lwe-CAR-T lwesihlanu oluvunywe yi-FDA.Umgaqo wechiza kukuvakalisa i-chimeric BCMA receptors kwiiseli ze-T zesigulana ngokusebenzisa i-lentivirus-mediated gene modification in vitro.Unyango lokuphelisa iiseli ze-T ezingalungiswanga ngokwemfuza kwizigulana, kwaye emva koko kufakwe iiseli ze-T ezilungisiweyo, ezifuna zibulale iiseli zomhlaza ezibonisa i-BCMA kwizigulana.
(11) Libmeldy
INKAMPANI: Iphuhliswe yi-Orchard Therapeutics.
Ixesha lokuthengisa: Ivunywe yiManyano yaseYurophu ukuba ifakwe kuluhlu ngoDisemba ka-2020.
Iimpawu: Kunyango lwe-metachromatic leukodystrophy (MLD).
Amagqabantshintshi: I-Libmeldy lunyango lofuzo olusekwe kwi-autologous CD34+ seli eziguqulwe ngokwemfuza kwi-vitro yi-lentivirus.Idatha yeklinikhi ibonisa ukuba i-intravenous intravenous ye-Libmeldy inokuguqula ngokufanelekileyo ikhosi ye-MLD yokuqala xa kuthelekiswa nemoto enzima kunye nokukhubazeka kwengqondo kwizigulana ezingaphathwanga ezikwiminyaka efanayo.
(12) Benoda
Inkampani: Iphuhliswe nguWuXi Giant Nuo.
Ixesha lokuthengisa: Ivunywe ngokusesikweni yi-NMPA ngoSeptemba 2021.
Izibonakaliso: Unyango lwezigulane ezikhulileyo ezinokuphinda zibuyele okanye eziphikisayo ezinkulu ze-B-cell lymphoma (r / r LBCL) emva komgca wesibini okanye ngaphezulu kwe-systemic therapy.
Amagqabantshintshi: I-Beinoda lunyango lwemfuza ye-CD19 ye-CAR-T echasene ne-CD19, kwaye ikwayimveliso engundoqo yeNkampani ye-WuXi Juro.Yimveliso yesibini ye-CAR-T evunyiweyo e-China, ngaphandle kwe-relapsed / refractory enkulu ye-B-cell lymphoid WuXi Giant Nuo kwakhona uceba ukuphuhlisa inaliti ye-Regiorensai yonyango lwezinye iimpawu ezininzi, kuquka i-follicular lymphoma (FL), i-mantle cell lymphoma (MCL), i-lymphocytic leukemia (CLL) , i-lymphocytic leukemia engapheliyo (i-CLL), i-lymphocytic ye-lymphocytic ye-lymphoma ye-DLL ebanzi kunye ne-lymphoma ye-lymphoma ye-DLL ebanzi.
(13) I-CARYKTI
Inkampani: Imveliso yokuqala yeLegend Biotech evunyiweyo ukuba ithengiswe.
Ixesha lokuthengisa: Ivunywe yi-FDA yokuthengisa ngoFebruwari 2022.
Izibonakaliso: kunyango lwe-myeloma ephindaphindiweyo okanye i-refractory (R / R MM).
Amanqaku: I-CARVYKTI (i-ciltacabtagene autoleucel, i-Cilta-cel emfutshane) yi-CAR-T ye-cell immune gene yonyango kunye ne-antibodies ezimbini ze-domain ezijoliswe kwi-B-cell maturation antigen (BCMA).Idatha ibonisa ukuba i-CARVYKTI Kwizigulane ezine-myeloma ezininzi eziphindaphindiweyo okanye ezichasayo eziye zafumana iindlela ezine okanye ngaphezulu zonyango zangaphambili (kubandakanywa ne-proteasome inhibitors, i-immunomodulators kunye ne-anti-CD38 monoclonal antibodies), izinga lokuphendula ngokubanzi kwe-98% libonisiwe.
(14)Ewe
INKAMPANI: Iphuhliswe yi-Atara Biotherapeutics.
IKhomishini yaseYurophu (EC) yokuthengisa ngoDisemba 2022, lunyango lokuqala lwehlabathi lwe-T cell oluvunyiweyo ukuba luthengiswe.
Izibonakaliso: Njenge-monotherapy ye-Epstein-Barr virus (EBV)-enxulumene ne-post-transplantation lymphoproliferative disease (EBV + PTLD), izigulane ezifumana unyango kufuneka zibe ngabantu abadala kunye nabantwana abangaphezu kweminyaka engama-2 ubudala abaye bafumana ubuncinane olunye unyango lweziyobisi.
Amanqaku: I-Ebvallo i-allogeneic EBV-specific universal T-cell gene gene therapy ejolise kwaye iphelisa iiseli ezosulelwe yi-EBV ngendlela ekhawulelwe yi-HLA.Ukuvunywa kolu nyango kusekelwe kwiziphumo zesigaba esibalulekileyo sophando lwezonyango lwe-3, kwaye iziphumo zibonise ukuba i-ORR yeqela le-HCT kunye neqela le-SOT yi-50%.Izinga lokuxolelwa okupheleleyo (CR) laliyi-26.3%, i-partial remission (PR) izinga laliyi-23.7%, kwaye ixesha eliphakathi loxolelo (TTR) laliyienyanga ze-1.1.Kwizigulane ze-19 ezifumene ukuxolelwa, i-11 yayinexesha lokuphendula (DOR) ngaphezu kweenyanga ze-6.Ukongezelela, ngokwemiqathango yokhuseleko, akukho zithintelo ezimbi ezifana ne-graft-versus-host disease (GvHD) okanye i-Ebvallo-related cytokine release syndrome eyenzekayo.
2. Unyango lwe-vivo gene olusekelwe kwii-viral vectors
(1) Gendicine/Jin Sheng
Inkampani: Iphuhliswe yiNkampani yaseShenzhen Saibainuo.
Ixesha lokuthengisa: Ivunyiwe ukuba idweliswe eTshayina ngo-2003.
Iimpawu: Kunyango lwentloko kunye nentamo squamous cell carcinoma.
Qaphela: I-Recombinant human p53 i-adenovirus inaliti i-Gendicine/Jinyousheng lichiza le-adenovirus vector gene therapy ichiza elinamalungelo azimeleyo epropathi enomgangatho ophezulu wokuqonda ephethwe yiNkampani yaseShenzhen Saibainuo.Uhlobo lwabantu lwe-adenovirus ye-5 luqulunqwe ngohlobo lwe-adenovirus yabantu 5. I-presentity yesakhiwo esiphambili sempembelelo ye-anti-tumor yeyeza, kwaye eyona nto yokugqibela isebenza njenge-carrier.I-adenovirus vector ithwala i-gene yonyango ye-p53 kwiseli ekujoliswe kuyo, ichaza i-tumor suppressor gene p53 kwiseli ekujoliswe kuyo, kunye nentetho yayo yemfuza Imveliso inokulawula iindidi ezininzi zejeni ezichasene nomhlaza kunye nokulawula imisebenzi yeentlobo ezahlukeneyo ze-oncogenes, ngaloo ndlela iphucula isiphumo sokubulala i-tumor ye-tumor sippressor.
(2) Rigvir
Inkampani: Iphuhliswe yiNkampani yaseLatima, eLatvia.
Ixesha lokudweliswa: Kuvunyiwe ukuba zidweliswe eLatvia ngowama-2004.
Iimpawu: Kunyango lwe-melanoma.
Amagqabantshintshi: I-Rigvir lunyango lwemfuza olusekwe kwi-ECHO-7 ye-enterovirus vector eguqulwe ngokwemfuza.Okwangoku, ichiza liye lamkelwa eLatvia, e-Estonia, ePoland, eArmenia, eBelarus, njalo njalo, kwaye liphantsi kobhaliso lwe-EMA kumazwe e-EU.Iimeko zonyango kule minyaka ilishumi idlulileyo zibonakalise ukuba intsholongwane ye-Rigvir oncolytic ikhuselekile kwaye iyasebenza, kwaye inokunyusa izinga lokusinda kwezigulane ze-melanoma ngamaxesha ama-4-6.Ukongeza, olu nyango lukwasebenza kwezinye iintlobo zomhlaza, kubandakanya umhlaza we-colorectal, umhlaza wepancreatic, umhlaza wesinyi, umhlaza wezintso, umhlaza wedlala lobudoda, umhlaza wemiphunga, umhlaza wesibeleko, i-lymphosarcoma, njl.
(3) I-Oncorine
Inkampani: Iphuhliswe yi Shanghai Sanwei Biological Company.
Ixesha lokuthengisa: Ivunyiwe ukuba idweliswe eTshayina ngo-2005.
Iimpawu: unyango lwentloko kunye nentamo amathumba, umhlaza wesibindi, umhlaza pancreatic, umhlaza wesibeleko kunye nezinye zomhlaza.
Amagqabantshintshi: I-Oncorine (安科瑞) yimveliso yonyango ye-oncolytic yentsholongwane esebenzisa i-adenovirus njengomphathi.I-adenovirus ye-oncolytic ifunyenwe, enokuthi iphindaphinde ngokuthe ngqo kwi-p53 yemfuza enqongopheleyo okanye engaqhelekanga, ekhokelela kwi-lysis yeeseli zethumba, ngaloo ndlela ibulala iiseli zethumba.ngaphandle kokonakalisa iiseli eziqhelekileyo.Uphononongo lweklinikhi lubonise ukuba i-Ankerui inokhuseleko oluhle kunye nokusebenza kakuhle kwiindidi ze-tumor ezimbi.
(4) Glybera
Inkampani: Iphuhliswe yi-uniQure.
Ixesha lokuthengisa: Ivunyiwe ukuba idweliswe eYurophu ngo-2012.
Izibonakaliso: Unyango lwe-lipoprotein lipase deficiency (LPLD) eneziqephu ezinzima okanye eziphindaphindiweyo ze-pancreatitis nangona ukutya okunamafutha okuthintelwe ngokungqongqo.
Amanqaku: I-Glybera (i-alipogene tiparvovec) isilwanyana sonyango olusekelwe kwi-AAV, esebenzisa i-AAV njengomphathi wokuhambisa i-LPL yonyango kwiiseli ze-muscle, ukwenzela ukuba iiseli ezihambelanayo zikwazi ukuvelisa inani elithile le-lipoprotein lipase, Ukunciphisa isifo, olu nyango lusebenza ixesha elide ixesha elide (i-drug effect) inokuthi ihlale ixesha elide (i-drug effect).Ichiza lihoxiswe kwimarike ngo-2017. Isizathu sokuhoxiswa kwayo sinokuhambelana nezinto ezimbini: ixabiso eliphezulu kunye nemfuno elinganiselwe yemarike.I-avareji yeendleko zonyango lwechiza liphezulu ukuya kutsho kwi-US$1 yesigidi, kwaye sisigulane esinye kuphela esithengileyo saza salisebenzisa ukuza kuthi ga ngoku.Nangona inkampani yeinshorensi yezonyango iyibuyisele i-US$900,000 ngayo, ikwangumthwalo omkhulu kwinkampani yeinshurensi.Ukongezelela, izibonakaliso ezijoliswe kuyo ichiza zinqabile kakhulu, kunye nezinga lezehlo malunga ne-1 kwi-1 yezigidi kunye nezinga eliphezulu lokuxilongwa kakubi.
(5) Imlygic
Inkampani: Iphuhliswe ngu-Amgen.
Ixesha lokuthengisa: Ngo-2015, yamkelwa ukuba idweliswe e-United States kunye ne-European Union.
Iimpawu: Unyango lwezilonda zemelanoma ezingenakususwa ngokupheleleyo ngotyando.
Amagqabantshintshi: I-Imlygic yintsholongwane ye-herpes simplex ethotyiweyo yohlobo 1 oluye lwatshintshwa bubuchwepheshe bemfuza (ukucima i-ICP34.5 kunye ne-ICP47 gene fragments, kwaye ifaka i-granulocyte macrophage colony-stimulating factor GM-CSF gene kwi-virus) (HSV-1) i-oncolytic virus virus yi-virus ye-oncolytic approve yokuqala kwi-FDA.Indlela yokulawula i-intralesional injection, enokuthi ifakwe ngokuthe ngqo kwizilonda ze-melanoma ukuze kubangele ukuphuka kweeseli ze-tumor, ukukhulula i-antigens ephuma kwi-tumor kunye ne-GM-CSF, kwaye ikhuthaze iimpendulo ze-immune anti-tumor.
(6) Luxturna
Inkampani: Iphuhliswe yi-Spark Therapeutics, i-subsidiary ye-Roche.
Ixesha lokuthengisa: Yavunywa ukuba ithengiswe yi-FDA kwi-2017, kwaye emva koko yamkelwa ukuthengisa eYurophu ngo-2018.
Izibonakaliso: Kunyango lwabantwana kunye nabantu abadala abalahlekelwe ngumbono ngenxa yekopi ephindwe kabini yokuguqulwa kofuzo lwe-RPE65 kodwa bagcine inani elaneleyo leeseli ze-retinal ezisebenzayo.
Amagqabantshintshi: I-Luxturna lunyango lofuzo olusekwe kwi-AAV olulawulwa ngesitofu esingaphantsi kwe-subretinal.Unyango lwe-gene lisebenzisa i-AAV2 njengomthwali wokuzisa ikopi esebenzayo ye-RPE65 gene yesiqhelo kwiiseli ze-retinal zesigulane, ukwenzela ukuba iiseli ezihambelanayo zibonise iprotheni ye-RPE65 eqhelekileyo, eyenza i-RPE65 yeprotheni yesigulane, ngaloo ndlela iphucula umbono wesigulane.
(7) Zolgensma
Inkampani: Iphuhliswe yi-AveXis, i-subsidiary ye-Novartis.
Ixesha lokuthengisa: Ivunywe yi-FDA yokuthengisa ngoMeyi ka-2019.
Izibonakaliso: Unyango lwe-Spinal Muscular Atrophy (SMA) izigulane ezingaphantsi kweminyaka eyi-2 ubudala.
Amagqabantshintshi: I-Zolgensma lunyango lwemfuza olusekelwe kwi-AAV vector.Eli chiza kuphela kwesicwangciso sonyango lwexesha elinye le-spinal muscular atrophy evunyiweyo ukuba ithengiswe kwihlabathi.Ukuqaliswa kweyeza kuvula ixesha elitsha kunyango lwe-spinal muscular atrophy.iphepha, yinkqubela phambili.Olu nyango lwe-gene lisebenzisa i-scAAV9 vector ukuzisa i-gene ye-SMN1 eqhelekileyo kwisigulane ngokusebenzisa i-intravenous infusion ukuvelisa iprotheyini ye-SMN1 eqhelekileyo, ngaloo ndlela iphucula umsebenzi weeseli ezichaphazelekayo ezifana ne-motor neurons.Ngokwahlukileyo koko, iziyobisi ze-SMA uSpinraza kunye ne-Evrysdi zifuna i-dosing ephindaphindiweyo yexesha elide.I-Spinraza inikwa ngesitofu somqolo rhoqo emva kweenyanga ezine, kwaye i-Evrysdi lichiza elithathwa ngomlomo imihla ngemihla.
(8) I-Delytact
Inkampani: Iphuhliswe nguDaiichi Sankyo Company Limited (TYO: 4568).
Ixesha lokuthengisa: Imvume enemiqathango evela kuMphathiswa wezeMpilo waseJapan, uMsebenzi neNtlalontle (MHLW) ngoJuni ka-2021.
Iimpawu: Kunyango lwe-glioma enobungozi.
Amagqabantshintshi: I-Delytact yimveliso yesine yonyango lwentsholongwane ye-oncolytic evunyiweyo kwihlabathi liphela, kunye nemveliso yokuqala ye-oncolytic yentsholongwane evunyiweyo kunyango lwe-glioma enobungozi.I-Delytact yintsholongwane ye-herpes simplex eyenziwe ngokwemfuza yohlobo loku-1 (HSV-1) oncolytic virus eyenziwe nguGqr Todo kunye noogxa.I-Delytact yazisa utshintsho olongezelelweyo lokucima kwi-G207 genome ye-HSV-1 yesizukulwana sesibini, iphucula ukuphindaphinda kwayo okukhethiweyo kwiiseli zomhlaza kunye nokungeniswa kweempendulo ze-anti-tumor zokukhusela ngelixa kugcinwa ukhuseleko oluphezulu.I-Delytact sisizukulwana sokuqala sesithathu se-oncolytic HSV-1 ngoku siphantsi kovavanyo lwezonyango.Ukwamkelwa kweDelytact eJapan kusekelwe ikakhulu kulingo lwezonyango lwengalo enye yesigaba sesi-2.Kwizigulane ezine-glioblastoma ephindaphindiweyo, i-Delytact iphumelele isiphelo sokuqala sonyaka omnye wokuphila, kwaye iziphumo zibonise ukuba i-Delytact ibonise ukusebenza kakuhle xa kuthelekiswa ne-G207.Amandla anamandla okuphindaphinda kunye nomsebenzi ophezulu we-antitumor.Oku kwakusebenzayo kwiimodeli zethumba elomeleleyo lebele, i-prostate, i-schwannomas, i-nasopharyngeal, i-hepatocellular, i-colorectal, i-peripheral nerve sheath tumors, kunye nomhlaza we-thyroid.
(9) Upstaza
INKAMPANI: Iphuhliswe yi-PTC Therapeutics, Inc. (NASDAQ: PTCT).
Ixesha lokuthengisa: Ivunywe yiManyano yaseYurophu ukuba ithengiswe ngoJulayi 2022.
Iimpawu: Ukusilela kwe-L-amino acid decarboxylase (AADC) enevumba elimnandi, ivunyiwe kunyango lwezigulane ezineminyaka eyi-18 nangaphezulu.
Amanqakwana: I-Upstaza™ (eladocagene exuparvovec) lunyango lwejini ye-vivo olune-adeno-associated virus uhlobo 2 (AAV2) njengomthwali.Izigulane ziyagula ngenxa yokuguqulwa kofuzo kwi-encoding enzyme ye-AADC.I-AAV2 iphethe ijini esempilweni efaka i-enzyme ye-AADC.Ifomu yembuyekezo yemfuza ifezekisa umphumo wonyango.Ngokwethiyori, ulawulo olunye lusebenza ixesha elide.Lunyango lokuqala oluthengisiweyo lwemfuza olutofwa ngokuthe ngqo kwingqondo.Ugunyaziso lokuthengisa lusebenza kuwo onke amazwe angamalungu e-EU angama-27, kunye ne-Iceland, iNorway kunye neLiechtenstein.
(10) Roctavian
Inkampani: Iphuhliswe yi-BioMarin Pharmaceutical (BioMarin).
Ixesha lokuthengisa: Ivunywe ukuba ithengiswe yi-European Union ngo-Agasti ka-2022;isigunyaziso sentengiso yi-UK Medicines and Healthcare products Administration (MHRA) ngoNovemba 2022.
Iimpawu: Kunyango lwezigulane zabantu abadala abane-hemophilia enzima A abangenayo imbali ye-FVIII factor inhibition kunye ne-negative kwi-AAV5 antibodies.
Amanqaku: I-Roctavian (valoctocogene roxaparvovec) isebenzisa i-AAV5 njenge-vector kwaye isebenzisa umgqugquzeli okhethekileyo wesibindi somntu we-HLP ukuqhuba ukubonakaliswa kwe-human coagulation factor VIII (FVIII) kunye ne-B domain ecinyiwe.Isigqibo seKomishoni yaseYurophu yokuvuma ukuthengiswa kwe-valoctocogene roxaparvovec isekelwe kwidatha epheleleyo yeprojekthi yophuhliso lwezonyango lwechiza.Phakathi kwabo, iziphumo zesigaba sesi-III solingo lwezonyango iGENER8-1 lubonise ukuba xa kuthelekiswa neenkcukacha zonyaka ngaphambi kokubhaliswa, emva kokufakwa okukodwa kwe-valoctocogene roxaparvovec, izinga lokuphuma kwegazi lonyaka (ABR) lincitshiswe kakhulu, ukuphindaphinda kokusetyenziswa kwe-recombinant coagulation factor VIII (F8) amalungiselelo eprotheyini ayancitshiswa, okanye umsebenzi wegazi we-F8 unyuke kakhulu emzimbeni.Emva kweeveki ze-4 zonyango, umlinganiselo wokusetyenziswa kwe-F8 yonyaka wesifundo kunye ne-ABR efuna unyango yancitshiswa nge-99% kunye ne-84%, ngokulandelanayo, kwaye umehluko wawubaluleke kakhulu (p <0.001).Iprofayili yokhuseleko yayilungile, kwaye akukho sifundo esifumene i-F8 factor inhibition, i-malignancy okanye i-thrombosis side effects, kwaye akukho ziganeko ezimbi kakhulu ezinxulumene nonyango (SAEs) zichazwe.
(11) Hemgenix
Inkampani: Iphuhliswe yi-UniQure Corporation.
Ixesha lokuthengisa: Ivunywe yi-FDA ukuze ithengiswe ngoNovemba ka-2022.
Iimpawu: Kunyango lwabaguli abadala abane-hemophilia B.
Amanqaku: I-Hemgenix lunyango lwemfuza olusekwe kwi-AAV5 vector.Ichiza lixhotyiswe nge-coagulation factor IX (FIX) ye-gene ye-FIX-Padua, elawulwa nge-intravenously.Emva kokulawula, i-gene inokubonakalisa i-FIX coagulation factor kwisibindi kwaye ifihla Emva kokungena egazini ukwenza umsebenzi wokudibanisa, ukuze kuphunyezwe injongo yokonyango, ithiyori, ulawulo olulodwa lusebenza ixesha elide.
(12) Adstiladrin
Inkampani: Iphuhliswe ngabakwaFerring Pharmaceuticals.
Ixesha lokuthengisa: Ivunywe yi-FDA ukuze ithengiswe ngoDisemba ka-2022.
Izibonakaliso: Kunyango lomhlaza wesinyi (NMIBC) ongaphenduliyo kwi-Bacillus Calmette-Guerin (BCG) .
Amanqaku: I-Adstiladrin lunyango lofuzo olusekwe kwi-adenoviral vector engaphindiyo, enokuthi igqithise i-interferon alfa-2b protein kwiiseli ekujoliswe kuzo, kwaye ilawulwa ngecatheter yomchamo kwi-bladder (ilawulwa kanye kwiinyanga ezintathu), iVector yentsholongwane inokosulela ngokufanelekileyo kwiiseli ze-bladder kunye ne-alfax-2 yeprotein. isiphumo se-utic.Le ndlela intsha yonyango lwemizila yemfuza ngaloo ndlela iguqula iiseli zodonga lwesinyi somguli zibe “ngumzi-mveliso” omncinane ovelisa i-interferon, ngaloo ndlela uphucula amandla omguli okulwa nomhlaza.
Ukhuseleko kunye nokusebenza kakuhle kwe-Adstiladrin kwavavanywa kwisifundo seklinikhi eninzi kuquka izigulane ze-157 ezinomngcipheko ophezulu we-BCG-engaphenduliyo i-NMIBC.Izigulane zifumana i-Adstiladrin rhoqo kwiinyanga ezintathu ukuya kwiinyanga ze-12, okanye de kube yityhefu engamkelekanga kunyango okanye ukuphindaphinda kwe-NMIBC ephezulu.Ngokubanzi, i-51 yepesenti yezigulane ezibhalisiweyo eziphathwe nge-Adstiladrin zifumene impendulo epheleleyo (ukungabikho kwazo zonke iimpawu zomhlaza ezibonwa kwi-cystoscopy, i-biopsy tissue, kunye nomchamo).
3. Iziyobisi ezincinci ze-nucleic acid
(1) Vitravene
Inkampani: Iphuhliswe ngokudibeneyo ngu-Ionis Pharma (owayesakuba ngu-Isis Pharma) kunye noNovartis.
Ixesha lokuthengisa: Kwi-1998 kunye ne-1999, yavunywa ukuba ithengiswe yi-FDA kunye ne-EU EMA.
Izibonakaliso: Kunyango lwe-cytomegalovirus retinitis kwizigulane ezine-HIV.
Amanqaku: I-Vitravene yi-antisense oligonucleotide ichiza, eyona nto yokuqala ye-oligonucleotide ichiza evunyiweyo ukuthengisa kwihlabathi.Kwinqanaba lokuqala loluhlu, imfuno yemarike yamachiza e-anti-CMV yayingxamiseke kakhulu;kamva, ngenxa yophuhliso lwe-antiretroviral esebenzayo kakhulu, inani leemeko ze-CMV lehla ngokukhawuleza.Ngenxa yokudodobala kwemfuno yemarike, ichiza lasungulwa ngo-2002 kunye no-2006 Ukurhoxiswa kumazwe e-EU nase-United States.
(2) Macugen
Inkampani: Iphuhliswe ngokudibeneyo yiPfizer kunye ne-Eyetech.
Ixesha lokuthengisa: Ivunyiwe ukuba ifakwe e-United States ngo-2004.
Iimpawu: Kunyango lwe-neovascular age-related macular degeneration.
Amanqaku: I-Macugen yi-pegylated modified oligonucleotide drug, enokuthi ijolise kwaye ibophe i-vascular endothelial growth factor (VEGF165 subtype), kunye nendlela yokulawula i-intravitreal injection.
(3) Defitelio
Inkampani: Iphuhliswe yiJazz Pharmaceuticals.
Ixesha lokuthengisa: Yavunywa ukuba ithengiswe yi-European Union kwi-2013 kwaye ivunywe yi-FDA yokuthengisa ngo-Matshi 2016.
Iimpawu: Kunyango lwesifo se-hepatic veno-occlusive ezinxulumene ne-renal okanye i-pulmonary dysfunction emva kwe-hematopoietic stem cell transplantation.
Amagqabantshintshi: I-Defitelio lichiza le-oligonucleotide, elingumxube we-oligonucleotides kunye neempawu ze-plasmin.Irhoxiwe kwimarike ngo-2009 ngenxa yezizathu zorhwebo.
(4) Kynamro
Inkampani: Iphuhliswe ngokudibeneyo ngu-Ionis Pharma kunye neKastle.
Ixesha lokuthengisa: Ngo-2013, yamkelwa ukuba ithengiswe eUnited States njengechiza lentandane.
Iimpawu: Kunyango lwe-adjuvant ye-homozygous yosapho lwe-hypercholesterolemia.
Ukuphawula: I-Kynamro yi-antisense oligonucleotide ichiza, eyi-oligonucleotide ye-antisense ejolise kwi-apo yabantu B-100 mRNA.I-Kynamro ilawulwa njenge-200 mg ngaphantsi kwesikhumba kanye ngeveki.
(5) Spinraza
Inkampani: Iphuhliswe yi-Ionis Pharmaceuticals.
Ixesha lokuthengisa: Ivunywe yi-FDA yokuthengisa ngoDisemba 2016.
Izibonakaliso: Kunyango lwe-spinal muscular atrophy (SMA).
Amagqabantshintshi: I-Spinraza (nusinersen) sisiyobisi esichasene ne-oligonucleotide.Ngokuzibophelela kwindawo ye-cleavage ye-SMN2 exon 7, i-Spinraza inokutshintsha i-RNA cleavage ye-SMN2 gene, ngokwandisa ukuveliswa kweprotheni ye-SMN esebenza ngokupheleleyo.Ngo-Agasti 2016, i-BIOGEN isebenzise ukhetho lwayo lokufumana amalungelo ehlabathi kwi-Spinraza.I-Spinraza yaqala kuphela ulingo lwayo lokuqala lweklinikhi kubantu kwi-2011. Kwiminyaka eyi-5 nje, yavunywa yi-FDA yokuthengisa kwi-2016, ebonisa ukuqaphela ngokupheleleyo kwe-FDA ukusebenza kwayo.Ichiza livunyiwe ukuthengisa e-China ngo-Ephreli 2019. Umjikelezo wonke wokuvunywa kwe-Spinraza e-China wawungaphantsi kweenyanga ze-6, kwaye yayiyiminyaka eyi-2 kunye neenyanga ezi-2 ukususela ekubeni i-Spinraza yavunywa okokuqala e-United States.Isantya soluhlu eTshayina sele sikhawuleza kakhulu.Oku kukwangenxa yokuba iZiko loVavanyo lweZiyobisi likhuphe "Isaziso sokuPapasha uluhlu lweBatch yokuQala yaMachiza aMatsha aphesheya afuneka ngokungxamisekileyo kwiKlinikhi yokuSebenza" ngoNovemba 1, 2018, kwaye yafakwa kwibhetshi yokuqala ye-40 yamachiza amatsha angaphandle ukuphononongwa okukhawulezileyo, phakathi kwayo i-Spinraza yafakwa kwindawo.
(6) IiEksodus 51
Inkampani: Iphuhliswe yi-AVI BioPharma (kamva yathiywa ngokuba yiSarepta Therapeutics).
Ixesha lokuthengisa: NgoSeptemba 2016, yavunywa ukuba ithengiswe yi-FDA.
Iimpawu: Kunyango lwe-Duchenne muscular dystrophy (DMD) kunye ne-exon 51 yokutsiba ukuguqulwa kofuzo kwijini ye-DMD.
Amanqaku: I-Exondys 51 yi-antisense oligonucleotide drug, i-oligonucleotide ye-antisense inokubophelela kwindawo ye-exon 51 ye-pre-mRNA ye-gene ye-DMD, okubangelwa ukubunjwa kwe-mRNA evuthiweyo, inxalenye ye-exon 51 ivaliwe I-Excision, ngaloo ndlela ilungisa ngokuyinxenye i-syndrome ye-protein ye-protein ye-syndrome, inceda ngokuyinxenye i-syndrome ye-protein ye-protein ye-mRNA. , ngaloo ndlela kuphucula iimpawu zesigulana.
(7) Tegsedi
Inkampani: Iphuhliswe yi-Ionis Pharmaceuticals.
Ixesha lokuthengisa: Yavunywa ukuba ithengiswe yi-European Union ngoJulayi ka-2018.
Iimpawu: Kunyango lwe-hereditary transthyretin amyloidosis (hATTR).
Amagqabantshintshi: I-Tegsedi lichiza le-antisense oligonucleotide elijolise kwi-transthyretin mRNA.Lichiza lokuqala elivunyiweyo kwihlabathi kunyango lwe-hATTR.Ilawulwa ngenaliti engaphantsi kwesikhumba.Ichiza linciphisa ukuveliswa kweprotheyini ye-ATTR ngokujolisa kwi-mRNA ye-transthyretin (ATTR), kwaye inomlinganiselo omhle wenzuzo-umngcipheko kunyango lwe-ATTR, kwaye i-neuropathy yesigulana kunye nomgangatho wobomi ziye zaphuculwa kakhulu, kwaye ziyahambelana neentlobo ze-TTR zokuguqula, Akukho nqanaba lesifo okanye ubukho be-cardiomyopathy.
(8) Onpattro
Inkampani: Iphuhliswe ngokudibeneyo yi-Alnylam Corporation kunye ne-Sanofi Corporation.
Ixesha lokuthengisa: Ivunyiwe ukuba ifakwe e-United States ngo-2018.
Iimpawu: Kunyango lwe-hereditary transthyretin amyloidosis (hATTR).
Amanqaku: I-Onpattro iyiyeza le-siRNA elijolise kwi-transthyretin mRNA, eyanciphisa ukuveliswa kweprotheyini ye-ATTR kwisibindi kunye nokunciphisa ukuqokelela kwe-amyloid deposits kwi-peripheral nerves ngokujolisa kwi-mRNA ye-transthyretin (ATTR) , ngaloo ndlela iphucula kwaye inciphise iimpawu zesifo.
(9) Givlaari
Inkampani: Iphuhliswe yi-Alnylam Corporation.
Ixesha lokuthengisa: Ivunywe yi-FDA yokuthengisa ngoNovemba ka-2019.
Izibonakaliso: Kunyango lwe-acute hepatic porphyria (AHP) kubantu abadala.
Amagqabantshintshi: I-Givlaari lichiza le-siRNA, iyeza lesibini le-siRNA elivunyiweyo ukuthengiswa emva kwe-Onpattro.Indlela yolawulo yinaliti engaphantsi kwesikhumba.Ichiza lijolise kwi-mRNA yeprotheyini ye-ALAS1, kwaye unyango lwenyanga kunye no-Givlaari lunokunciphisa ngokuphawulekayo nangokusisigxina inqanaba le-ALAS1 esibindini, ngokunciphisa amanqanaba e-neurotoxic ALA kunye ne-PBG kuluhlu oluqhelekileyo, ngaloo ndlela Ukunciphisa iimpawu zesifo sesigulane.Idatha ibonise ukuba izigulane eziphathwe nge-Givlaari zine-74% yokunciphisa inani lokubamba xa kuthelekiswa neqela le-placebo.
(10) IiVyondys53
INKAMPANI: Iphuhliswe yiSarepta Therapeutics.
Ixesha lokuthengisa: Ivunywe yi-FDA yokuthengisa ngoDisemba ka-2019.
Iimpawu: Kunyango lwezigulane ze-DMD ezine-dystrophin gene exon 53 splicing mutation.
Amagqabantshintshi: I-Vyondys 53 ichiza le-oligonucleotide ye-antisense, ejolise kwinkqubo yokudibanisa i-dystrophin pre-mRNA.I-Exon 53 inqunyulwe ngokuyinxenye, okt ayikho kwi-mRNA evuthiweyo, kwaye yenzelwe ukuvelisa i-dystrophin ecuthiweyo kodwa esebenzayo, ngaloo ndlela iphucula umthamo wokuzilolonga kwizigulana.
(11) Waylivra
Inkampani: Iphuhliswe yi-Ionis Pharmaceuticals kunye ne-Akcea Therapeutics encedisayo.
Ixesha lokuthengisa: Yavunywa ukuba ithengiswe yi-Arhente yaMayeza yaseYurophu (EMA) ngoMeyi ka-2019.
Izibonakaliso: Njengonyango lwe-adjuvant ukongeza kulawulo lokutya kwizigulane zabantu abadala abane-familial chylomicronemia syndrome (FCS).
Amagqabantshintshi: I-Waylivra lichiza le-antisense oligonucleotide, lichiza lokuqala elivunyiweyo ukuba lithengiswe kwihlabathi kunyango lwe-FCS.
(12) Leqvio
Inkampani: Iphuhliswe nguNovartis.
Ixesha lokuthengisa: Ivunywe yiManyano yaseYurophu ukuba ithengiswe ngoDisemba ka-2020.
Izibonakaliso: Kunyango lwabantu abadala abane-hypercholesterolemia yokuqala (i-heterozygous yentsapho kunye ne-non-familial) okanye i-dyslipidemia edibeneyo.
Amagqabantshintshi: I-Leqvio lichiza le-siRNA elijolise kwi-PCSK9 mRNA.Lunyango lokuqala lwehlabathi lwe-siRNA lokuthoba i-cholesterol (LDL-C).Ilawulwa ngenaliti engaphantsi kwesikhumba.Ichiza linciphisa izinga leprotheni ye-PCSK9 ngokuphazamiseka kwe-RNA, ngaloo ndlela inciphisa izinga le-LDL-C.Idatha yezonyango ibonisa ukuba kwizigulana ezingakwaziyo ukunciphisa amanqanaba e-LDL-C ukuya kumlinganiselo ekujoliswe kuwo emva konyango ngowona mlinganiselo uphezulu onyanyezelweyo weestatins, iLeqvio inokunciphisa iLDL-C malunga nama-50%.
(13) Uxolo
Inkampani: Iphuhliswe yi-Alnylam Pharmaceuticals.
Ixesha lokuthengisa: Ivunywe yiManyano yaseYurophu ukuba ithengiswe ngoNovemba ka-2020.
Izibonakaliso: Kunyango lwe-hyperoxaluria yokuqala yohlobo lwe-1 (PH1).
Amanqaku: I-Oxlumo iyichiza le-siRNA elijolise kwi-hydroxyacid oxidase 1 (HAO1) mRNA, kwaye indlela yokulawula i-injection subcutaneous injection.Ichiza laphuhliswa kusetyenziswa ikhemistri yozinzo ephuculweyo ye-Alnylam yamva nje, i-ESC-GalNAc itekhnoloji yokudibanisa, eyenza ukuba i-siRNA ilawulwe ngaphantsi kwesikhumba ngokuzingisa okukhulu kunye namandla.Ichiza lithoba okanye linqande i-hydroxyacid oxidase 1 (HAO1) mRNA, inciphisa inqanaba le-glycolate oxidase esibindini, kwaye emva koko idle i-substrate efunekayo kwimveliso ye-oxalate, ukunciphisa ukuveliswa kwe-oxalate ukulawula ukuqhubela phambili kwesifo kwizigulane kunye nokuphucula iimpawu zesifo.
(14) Viltepso
Inkampani: Iphuhliswe ngu-NS Pharma, i-subsidiary ye-Nippon Shinyaku.
Ixesha lokuthengisa: Ivunywe yi-FDA yokuthengisa ngo-Agasti ka-2020.
Iimpawu: Kunyango lwe-Duchenne muscular dystrophy (DMD) kunye ne-exon 53 yokutsiba ukuguqulwa kofuzo kwijini ye-DMD.
Amanqaku: I-Viltepso yi-antisense oligonucleotide yeziyobisi ezinokubophelela kwindawo ye-exon 53 ye-pre-mRNA ye-DMD gene, ebangela ukuba inxalenye ye-exon 53 ikhutshwe emva kokubunjwa kwe-mRNA evuthiweyo, ngaloo ndlela ilungisa ngokuyinxenye isakhelo sokufunda se-mRNA Ibhokisi inceda izigulane ukuba zidibanise ezinye iifom zeprotheni eziphuculweyo ze-dystrophin ezimfutshane, apho izigulane ziphucula iifom zeprotheni ezimfutshane.
(15) IiAmondi ezingama-45
Inkampani: Iphuhliswe yiSarepta Therapeutics.
Ixesha lokuthengisa: Ivunywe yi-FDA yokuthengisa ngoFebruwari 2021.
Iimpawu: Kunyango lwe-Duchenne muscular dystrophy (DMD) kunye ne-exon 45 yokutsiba ukuguqulwa kofuzo kwijini ye-DMD.
Amanqaku: I-Amondys 45 yi-antisense oligonucleotide drug, i-oligonucleotide ye-antisense inokubophelela kwindawo ye-exon 45 ye-pre-mRNA ye-gene ye-DMD, okubangela ukuba inxalenye ye-exon 45 ivalwe emva kokubunjwa kwe-mRNA evuthiweyo Excision, ngaloo ndlela i-dysphise ye-frames yesiqhelo ilungisa i-syphilis ye-dystro esebenza ngokuyinxenye, inceda i-mRNA i-functional framework, inceda i-mRNA i-functional framework. iprotheni, ngaloo ndlela iphucula iimpawu zesigulane.
(16) Amvuttra (vutrisiran)
Inkampani: Iphuhliswe yi-Alnylam Pharmaceuticals.
Ixesha lokuthengisa: Ivunywe yi-FDA ukuze ithengiswe ngoJuni ka-2022.
Izibonakaliso: Kunyango lwe-hereditary transthyretin amyloidosis kunye ne-polyneuropathy (hATTR-PN) kubantu abadala.
Amanqakwana: I-Amvuttra (Vutrisiran) lichiza le-siRNA elijolise kwi-transthyretin (ATTR) mRNA, elawulwa ngesitofu esingaphantsi kwesikhumba.I-Vutrisiran isekelwe kwi-Alnylam's Enhanced Stability Chemistry (ESC)-GalNAc iplatifomu yokuhanjiswa kweplatifomu yokudibanisa kunye nokwandisa amandla kunye nokuzinza kwe-metabolic.Ukuvunywa kwonyango kusekelwe kwiinyanga ze-9 zedatha yeSigaba sesi-III sophando lweklinikhi (HELIOS-A), kwaye iziphumo ezipheleleyo zibonisa ukuba unyango luphucule iimpawu ze-hATTR-PN, kwaye ngaphezu kwe-50% yemeko yezigulane yatshintshwa okanye yamiswa ukuba ibe nzima.
4. Amanye amayeza onyango lwemfuza
(1) Rexin-G
Inkampani: Iphuhliswe yi-Epeius Biotech.
Ixesha lokuthengisa: Kwi-2005, yavunywa ukuba ithengiswe yi-Philippine Food and Drug Administration (BFAD).
Izalathisi: Kunyango lwemihlaza ephezulu ekwaziyo ukumelana nechemotherapy.
Amagqabantshintshi: I-Rexin-G yinaliti ye-nanoparticle enemfuza.Yazisa i-cyclin ye-G1 yejini eguqukayo kwiiseli ekujoliswe kuzo ngokusebenzisa i-retroviral vector ukubulala ngokukodwa amathumba aqinileyo.Indlela yokulawula i-intravenous infusion.Njengechiza elijoliswe kwithumba elifuna ngenkuthalo kwaye litshabalalise iiseli zomhlaza we-metastatic, linesiphumo esithile sokunyanga kwizigulana ezingaphumeleliyo kwamanye amachiza omhlaza, kubandakanya ibhayoloji ekujoliswe kuyo.
(2) Neovasculgen
Inkampani: Iphuhliswe liziko le-Human stem cells.
Ixesha lokudweliswa: Lavunywa ukuba lifakwe eRashiya ngoDisemba 7, 2011, laza lamiselwa eUkraine ngo-2013.
Izibonakaliso: Kunyango lwe-peripheral vascular arterial disease, kubandakanywa ne-ischemia yelungu elibi.
Amagqabantshintshi: I-Neovasculgen lunyango lwemfuza olusekwe kwi-plasmids ye-DNA.I-vascular endothelial growth factor (VEGF) i-gene ye-165 yakhiwe kwi-backbone ye-plasmid kwaye ifakwe kwizigulane.
(3) Collategene
Inkampani: Iphuhliswe ngokudibeneyo yiYunivesithi yase-Osaka kunye neenkampani ze-capital venture.
Ixesha lokuthengisa: Ivunywe nguMphathiswa wezeMpilo, uMsebenzi kunye neNtlalontle yaseJapan ngo-Agasti ka-2019.
Iimpawu: Unyango lwe-ischemia ebalulekileyo esezantsi.
Amagqabantshintshi: I-Collategene lunyango lwemfuza olusekwe kwi-plasmid, iyeza lokuqala lonyango lwemfuza yasekhaya eliveliswe yi-AnGes, inkampani yonyango lwemfuza eJapan.Inxalenye ephambili yeli chiza yi-plasmid ehamba ze equlethe i-human hepatocyte factor factor (HGF) ulandelelwano lwemfuza.Ukuba ichiza litofelwe kwizihlunu zamalungu asezantsi, i-HGF echaziweyo iya kukhuthaza ukwakheka kwemithambo yegazi emitsha ejikeleze imithambo yegazi evalekileyo.Ulingo lwezonyango luye lwaqinisekisa umphumo walo ekuphuculeni izilonda.
I-Foregene ingalunceda njani uphuhliso lonyango lwemfuza?
Sinceda ukugcina ixesha lokuhlola kuvavanyo olukhulu, kwinqanaba lokuqala lophuhliso lwamachiza e-siRNA.
Iinkcukacha ezithe vetshe ndwendwela:
https://www.foreivd.com/cell-direct-rt-qpcr-kit-direct-rt-qpcr-series/
Ixesha lokuposa: Dec-27-2022