Kutshanje, amachiza amathathu onyango lwemfuza avunyiweyo ukuba athengiswe, angala: (1) NgoJulayi 21, 2022, iPTC Therapeutics, Inc. (NASDAQ: PTCT) yabhengeza ukuba unyango lwe-AAV gene therapy Upstaza™ luvunyiwe yiKomishoni yaseYurophu Lunyango lokuqala oluthengisiweyo lofuzo olufakwe ngqo kwingqondo ye-Gene ingqondo ivunyiwe ukuba ithengiswe).(2) Ngomhla we-17 ka-Agasti, i-2022, i-US Food and Drug Administration (FDA) yamkele unyango lwemfuza ye-Bluebird Bio's Zynteglo (betibeglogene autotemcel, beti-cel) kunyango lwe-beta thalassemia.Ukuvunywa kwonyango eUnited States ngokungathandabuzekiyo "uncedo kwikhephu" yeBluebird Bio, ekwingxaki yezemali.(3) Ngo-Agasti 24, 2022, i-BioMarin Pharmaceutical (BioMarin) yazisa ukuba iKhomishoni yaseYurophu ivume ukuthengiswa okunemiqathango yeROCTAVIAN™ (valoctocogene roxaparvovec), unyango lwemfuza ye-hemophilia A, kunyango lwezigulane ezingenambali ye-FVIII factor inhibitors kunye ne-negative AAV5 i-hemophilia i-hemophilia izigulane ze-hemophilia yangaphambili Unyango lwemfuza oluvunyiweyo ukuba luthengiswe).Ukuza kuthi ga ngoku, amayeza onyango lwemfuza angama-41 amkelwe ukuthengiswa kwihlabathi liphela.
I-Gene yiyunithi esisiseko yemfuza elawula iimpawu.Ngaphandle kwemizila yemfuza yezinye iintsholongwane eziyilwe yiRNA, imizila yemfuza yezinto ezininzi iyilwe yiDNA.Uninzi lwezifo zendalo zibangelwa kukunxibelelana phakathi kofuzo kunye nokusingqongileyo, kwaye izifo ezininzi zinokunyangwa okanye zithotywe ngokwenyani ngonyango lwemfuza.Unyango lweGene luthathwa njengenguquko kwicandelo lamayeza kunye nekhemesti.Amachiza onyango olubanzi lwemfuza lubandakanya iziyobisi ezisekelwe kwiDNA-modified DNA drug (ezifana nentsholongwane yentsholongwane-based in vivo gene therapy drugs, in vitro gene therapy drugs, drug plasmid ze, etc.) kunye neziyobisi RNA (ezifana neziyobisi oligonucleotide antisense, iziyobisi siRNA, kunye nonyango lwemfuza mRNA, njl.);amayeza onyango lwe-Gene achazwe ngokumxinwa abandakanya amachiza e-plasmid DNA, amachiza onyango lwemfuza asekelwe kwiintsholongwane zentsholongwane, amayeza onyango lwemfuza asekelwe kwiintsholongwane zebhaktiriya, iinkqubo zokuhlela imizila yemfuza kunye namachiza onyango lweseli aguqulwa ngokwemfuza kwi-vitro.Emva kweminyaka yophuhliso olubi, amayeza onyango lwemfuza afumene iziphumo ezikhuthazayo zeklinikhi.(ngaphandle kogonyo lwe-DNA kunye nogonyo lwe-mRNA), amachiza onyango lwemfuza angama-41 avunyiwe ukuba athengiswe kwihlabathi.Ngokusungulwa kweemveliso kunye nophuhliso olukhawulezayo lwetekhnoloji yonyango lwemfuza, unyango lwemfuza sele luza kungenisa ixesha lophuhliso olukhawulezayo.
Ukuhlelwa konyango lwemfuza (Umthombo womfanekiso: Biological Jingwei)
Eli nqaku lidwelisa i-41 yemfuza yonyango evunyiweyo ukuba ithengiswe (ngaphandle kogonyo lwe-DNA kunye nogonyo lwe-mRNA).
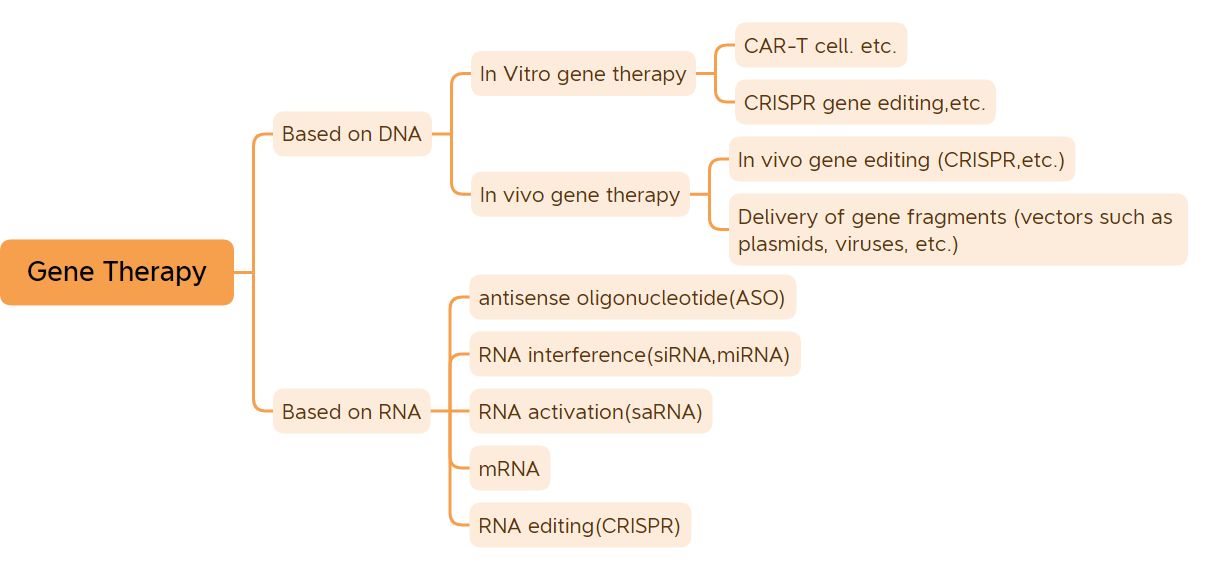
1. Unyango lwemfuza ye-in vitro
(1) Strimvelis
Inkampani: Iphuhliswe yiGlaxoSmithKline (GSK).
Ixesha lokuthengisa: Ivunywe yi-European Union ngoMeyi ka-2016.
Izibonakaliso: Kunyango lwe-immunodeficiency edibeneyo (SCID).
Amanqaku: Inkqubo eqhelekileyo yolu nyango kukuqala ukufumana iiseli ze-hematopoietic stem zesigulane, zandise kwaye zikhule kwi-vitro, kwaye emva koko usebenzise i-retrovirus ukwazisa ikopi ye-ADA esebenzayo (i-adenosine deaminase) yofuzo kwiiseli zabo ze-hematopoietic stem, kwaye ekugqibeleni udlulisele iiseli ze-hematopoietic stem eziguquliweyo.Iiseli ze-Hematopoietic stem ziphinda zifakwe emzimbeni.Iziphumo zeklinikhi zibonise ukuba izinga lokusinda leminyaka emi-3 yezigulane ze-ADA-SCID ezinyangwa nge-Strimvelis yi-100%.
(2) I-Zalmoxis
Inkampani: Iveliswe nguMolMed, eItali.
Ixesha lokuthengisa: Kufunyenwe isigunyaziso sentengiso esinemiqathango ye-EU ngo-2016.
Iimpawu: Isetyenziselwa unyango lwe-adjuvant ye-immune system yezigulane emva kokutshintshwa kwe-hematopoietic stem cell.
Amagqabantshintshi: I-Zalmoxis yi-allogeneic T cell suicide gene immunotherapy elungiswe yi-retroviral vector.I-1NGFR kunye ne-HSV-TK Mut2 iijini zokuzibulala zivumela abantu ukuba basebenzise i-ganciclovir nanini na ukuba babulale iiseli ze-T ezibangela iimpendulo ezichasene ne-immune, ukukhusela ukuwohloka okuqhubekayo kwe-GVHD enokuthi yenzeke, kunye nokubuyisela ukusebenza komzimba kwizigulane ezine-haploidentical HSCT emva kokuhlinzwa i-Escort.
(3) Invossa-K
Inkampani: Iphuhliswe yinkampani yeTissueGene (KolonTissueGene).
Ixesha lokuthengisa: Ivunyiwe ukuba ifakwe kuluhlu eMzantsi Korea ngoJulayi ka-2017.
Izibonakaliso: Kunyango lwe-arthrosis yamadolo.
Amagqabantshintshi: I-Invossa-K lunyango lwe-allogeneic cell gene olubandakanya iichondrocyte zabantu.Iiseli ze-allogeneic ziguqulwa ngokwezakhi zofuzo kwi-vitro, kwaye iiseli eziguquliweyo zinokuvakalisa kwaye zifihle ukuguqula ukukhula kwe-β1 (TGF-β1) emva kwe-injection ye-intra-articular.β1), ngaloo ndlela kuphuculwe iimpawu ze-osteoarthritis.Iziphumo zeklinikhi zibonisa ukuba i-Invossa-K inokuphucula kakhulu i-arthritis yamadolo.Ilayisenisi yarhoxiswa ngumlawuli weziyobisi waseMzantsi Korea ngo-2019 kuba umenzi ubhale ngokungalunganga izithako ezisetyenzisiweyo.
(4) Zynteglo
Inkampani: Iphuhliswe yinkampani ye-American bluebird bio (i-bluebird bio) inkampani.
Ixesha lokuthengisa: Ivunywe yiManyano yaseYurophu ngo-2019, kwaye yamkelwe yi-FDA ngo-Agasti ka-2022.
Iimpawu: Kunyango lwe-β-thalassemia exhomekeke kutofelo-gazi.
Amanqaku: I-Zynteglo yi-lentiviral in vitro gene gene therapy, esebenzisa i-lentiviral vector ukuzisa ikopi esebenzayo ye-gene ye-β-globin eqhelekileyo (i-βA-T87Q-globin gene) kwiiseli ze-hematopoietic stem ezisuswe kwizigulane., kwaye emva koko ifake ezi seli ze-autologous ze-hematopoietic stem ezitshintshiweyo zibuyele kwisigulana.Emva kokuba isigulane sine-gene ye-βA-T87Q-globin eqhelekileyo, inokuvelisa iprotheyini ye-HbAT87Q eqhelekileyo, enokunciphisa ngokufanelekileyo okanye yokuphelisa isidingo sokutofelwa igazi.Lunyango lwexesha elinye olwenzelwe ukutshintsha utofelo-gazi lobomi kunye namayeza obomi bonke kwizigulana ezineminyaka eli-12 ubudala nangaphezulu.
(5) Skysona
Inkampani: Iphuhliswe yinkampani ye-American bluebird bio (i-bluebird bio) inkampani.
Ixesha lokuthengisa: Ivunywe yi-EU ukuba ithengiswe ngoJulayi 2021.
Izibonakaliso: Kunyango lwe-cerebral adrenoleukodystrophy (CALD) yokuqala.
Amagqabantshintshi: Unyango lofuzo lwe-Skysona lolona nyango lofuzo lwexesha elinye oluvunyiweyo kunyango lwe-cerebral adrenoleukodystrophy (CALD) yokuqala.I-Skysona (elivaldogene autotemcel, Lenti-D) yi-hematopoietic stem cell lentiviral in vitro gene therapy therapy Lenti-D.Inkqubo eqhelekileyo yonyango ngolu hlobo lulandelayo: iiseli ze-autologous hematopoietic stem zithathwa kwisigulane, ziguqulelwe kwi-vitro yi-lentivirus ephethe i-gene ye-ABCD1 yomntu, kwaye iphinde ifakwe kwisigulane.Kunyango lwezigulane ezingaphantsi kweminyaka eyi-18 kunye ne-ABCD1 ye-gene mutation kunye ne-CALD.
(6) Kymriya
Inkampani: Iphuhliswe nguNovartis.
Ixesha lokuthengisa: Ivunywe yi-FDA ngo-Agasti ka-2017.
Izibonakaliso: Unyango lwe-precursor B-cell acute lymphoblastic leukemia (ALL) kunye ne-DLBCL ephindaphindiweyo kunye ne-refractory.
Amanqakwana: I-Kymriah lichiza lonyango lwe-lentiviral in vitro gene therapy, unyango lokuqala oluvunyiweyo lwe-CAR-T kwihlabathi, olujolise kwi-CD19 kunye nokusetyenziswa kwe-co-stimulatory factor 4-1BB.Ixabiso yi-$475,000 e-US kunye ne-$ 313,000 eJapan.
(7) Yescarta
Inkampani: Iphuhliswe nguKite Pharma, icandelo laseGiliyadi.
Ixesha lokuthengisa: Ivunywe yi-FDA ngo-Oktobha 2017.
Izalathiso: Kunyango lwe-lymphoma enkulu ebuyileyo okanye echasayo.
Amanqaku: I-Yescarta lunyango lwe-retroviral in vitro gene.Lunyango lwesibini lwe-CAR-T oluvunyiweyo kwihlabathi.Ijolise kwi-CD19 kwaye isebenzisa i-costimulatory factor ye-CD28.Amaxabiso e-US yi-373,000 yeedola.
(8) ITecartus
Inkampani: Iphuhliswe yiGiliyadi (GILD).
Ixesha lokuthengisa: Ivunywe yi-FDA ngoJulayi 2020.
Izalathisi: Kwi-mantle cell lymphoma ebuyele umva okanye irefractory.
Amanqaku: I-Tecartus i-autologous CAR-T cell therapy ejolise kwi-CD19, kwaye yonyango lwesithathu lwe-CAR-T oluvunyiweyo ukuthengiswa kwihlabathi.
(9) Breyanzi
Inkampani: Iphuhliswe nguBristol-Myers Squibb (BMS).
Ixesha lokuthengisa: Ivunywe yi-FDA ngoFebruwari 2021.
Izibonakaliso: I-Relapsed or refractory (R / R) enkulu ye-B-cell lymphoma (LBCL).
Amanqaku: I-Breyanzi yi-in vitro gene therapy esekelwe kwi-lentivirus, kunye nonyango lwesine lwe-CAR-T oluvunyiweyo ukuthengiswa kwihlabathi, lujolise kwi-CD19.Ukuvunywa kweBreyanzi sisiganeko esibalulekileyo seBristol-Myers Squibb kwinkalo ye-cellular immunotherapy, eyafunyanwa nguBristol-Myers xa ifumana iCelgene nge-74 yeebhiliyoni zeedola ngo-2019.
(10) Abecma
Inkampani: Iphuhliswe yiBristol-Myers Squibb (BMS) kunye ne-bluebird bio.
Ixesha lokuthengisa: Ivunywe yi-FDA ngoMatshi ka-2021.
Iimpawu: I-myeloma ephindaphindayo okanye echasayo.
Amagqabantshintshi: I-Abecma lunyango lwe-lentivirus olusekwe kwi-vitro gene, unyango lokuqala lwehlabathi lwe-CAR-T lweseli olujolise kwi-BCMA, kunye nonyango lwe-CAR-T lwesihlanu oluvunywe yi-FDA.Umgaqo wechiza kukuvakalisa i-chimeric BCMA receptor kwiiseli ze-T ezizihambelayo zesigulana ngokusebenzisa i-lentivirus-mediated genetic modification in vitro.Ngaphambi kokufakwa kweyeza le-gene yeseli, isigulane safumana iikhompawundi ezimbini ze-cyclophosphamide kunye ne-fludarabine yonyango lwangaphambili.Unyango lokususa iiseli ze-T ezingalungiswanga kwisigulana, kwaye emva koko kufakwe iiseli T ezilungisiweyo zibuyele emzimbeni wesigulana zikhangele kwaye zibulale iiseli zomhlaza ezibonisa i-BCMA.
(11) Libmeldy
Inkampani: Iphuhliswe yi-Orchard Therapeutics.
Ixesha lokuthengisa: Ivunywe yiManyano yaseYurophu ukuba ifakwe kuluhlu ngoDisemba ka-2020.
Iimpawu: Kunyango lwe-metachromatic leukodystrophy (MLD).
Amagqabantshintshi: I-Libmeldy lunyango lwemfuza olusekwe kwi-lentiviral in vitro gene modification of autologous CD34+ cells.Idatha yeklinikhi ibonisa ukuba i-intravenous infusion ye-Libmeldy iyasebenza ekuguquleni ikhosi ye-MLD yokuqala kunye ne-motor enzima kunye nokukhubazeka kwengqondo kwizigulana ezingaphathwanga ezikwiminyaka efanayo.
(12) Benoda
Inkampani: Iphuhliswe nguWuXi Junuo.
Ixesha lokuthengisa: Ivunywe ngokusesikweni yi-NMPA ngoSeptemba 2021.
Izibonakaliso: Unyango lwe-B-cell lymphoma enkulu ephindaphindiweyo okanye i-refractory (r / r LBCL) kwizigulane zabantu abadala emva komgca wesibini okanye unyango lwenkqubo.
Amagqabantshintshi: I-Benoda lunyango lofuzo lwe-CD19 ye-CAR-T, kwaye ikwayimveliso engundoqo ye-WuXi Junuo.Yimveliso yesibini ye-CAR-T evunyiweyo e-China, ngaphandle kwe-relapsed / refractory enkulu ye-B-cell lymphoma.Ukongeza, iWuXi Junuo ikwaceba ukuphuhlisa inaliti ye-Ruiki Orenza yonyango lwezinye izalathisi ezahlukeneyo, kubandakanya i-follicular lymphoma (FL), i-mantle cell lymphoma (MCL), i-lymphocytic leukemia engapheliyo (CLL), umgca wesibini wokusasaza i-B-cell lymphoma enkulu (DLBCL) kunye ne-acute lymphoblastic leukemia (YONKE).
(13) I-CARYKTI
Inkampani: Imveliso yokuqala evunyiweyo yeLegend Bio.
Ixesha lokuthengisa: Ivunywe yi-FDA ngoFebruwari 2022.
Izibonakaliso: Unyango lwe-myeloma ephindaphindiweyo okanye i-refractory (R / R MM).
Amanqaku: I-CARVYKTI (i-ciltacabtagene autoleucel, ebizwa ngokuba yi-Cilta-cel) yi-CAR-T ye-cell immune gene therapy kunye ne-antibodies ezimbini ze-domain ezijoliswe kwi-B cell maturation antigen (BCMA).Idatha ibonisa ukuba i-CARVYKTI ibonise izinga lokuphendula ngokubanzi ukuya kwi-98% kwizigulane ezine-myeloma ephindaphindiweyo okanye e-refractory eye yafumana unyango olune okanye ngaphezulu kwangaphambili, kubandakanywa i-proteasome inhibitors, i-immunomodulators, kunye ne-anti-CD38 monoclonal antibodies.
2. Unyango lwe-vivo gene olusekelwe kwii-viral vectors
(1) I-Gendicine / ukuzalwa kwakhona
Inkampani: Iphuhliswe yiNkampani yaseShenzhen Saibainuo.
Ixesha lokuthengisa: Ivunyiwe ukuba ifakwe kuluhlu e-China ngo-2003.
Iimpawu: Kunyango lwe-squamous cell carcinoma yentloko kunye nentamo.
Amanqakwana: Inaliti ye-adenovirus ye-Recombinant p53 yabantu i-Gendicine/Jinshengsheng lichiza lonyango lwe-adenovirus vector gene enamalungelo azimeleyo epropathi enomgangatho ophezulu wokuqonda ephethwe yiNkampani yaseShenzhen Saibainuo.Ichiza iqulunqwe eqhelekileyo human ithumba suppressor gene p53 kunye artificially modified recombinant replication-deficident Human adenovirus type 5 iqulunqwe uhlobo adenovirus yabantu 5. Eyangaphambili sisakhiwo engundoqo ichiza ukuba asebenzise isiphumo anti-ithumba, kwaye yokugqibela ikakhulu isebenza njengomthwali.I-adenovirus vector ithwala i-rapeutic gene p53 kwiseli ekujoliswe kuyo, kwaye ivakalisa i-tumor suppressor gene p53 kwiseli ekujoliswe kuyo.Imveliso inokunyusa-ukulawula iintlobo ezahlukeneyo ze-anti-cancer kunye nokunciphisa-ukulawula imisebenzi yee-oncogenes ezahlukeneyo, ngaloo ndlela iphucula impembelelo ye-anti-tumor yomzimba kunye nokufezekisa injongo yokubulala amathumba.
(2) Rigvir
Inkampani: Iphuhliswe yinkampani yaseLatvia iLatima.
Ixesha lokuthengisa: Iphunyezwe eLatvia ngo-2004.
Iimpawu: Kunyango lwe-melanoma.
Amagqabantshintshi: I-Rigvir lunyango lofuzo olusekwe kwi-gene-modified ECHO-7 enterovirus vector, esetyenziswe eLatvia, e-Estonia, ePoland, eArmenia, eBelarus nakwezinye iindawo, kwaye ikwabhaliswa kunye ne-EMA ye-European Union..Iimeko zonyango kule minyaka ilishumi idlulileyo zibonakalise ukuba intsholongwane ye-Rigvir oncolytic ikhuselekile kwaye iyasebenza, kwaye inokuphucula izinga lokusinda kwizigulane ze-melanoma ngamaxesha ama-4-6.Ukongeza, unyango lukwafanelekile kwezinye iintlobo zomhlaza, kubandakanya umhlaza we-colorectal, umhlaza wepancreatic, umhlaza wesinyi.umhlaza, umhlaza wezintso, umhlaza wedlala lobudoda, umhlaza wemiphunga, umhlaza wesibeleko, i-lymphosarcoma, njl.
(3) I-Oncorine/Ankerui
Inkampani: Iphuhliswe yiShanghai Sunway Biotechnology Co., Ltd.
Ixesha lokuthengisa: Ivunyiwe ukuba ifakwe kuluhlu e-China ngo-2005.
Iimpawu: Unyango lwamathumba entloko kunye nentamo, umhlaza wesibindi, umhlaza wepancreatic, umhlaza womlomo wesibeleko kunye neminye imihlaza.
Amagqabantshintshi: I-Oncorine yimveliso yonyango ye-oncolytic ye-virus esebenzisa i-adenovirus njenge-vector.I-oncolytic adenovirus efunyenweyo inokuphinda iphindaphindeke ngokukodwa kwi-tumor esweleyo okanye engaqhelekanga ye-p53 gene, ebangela i-tumor cell lysis, ngaloo ndlela ibulale iiseli zethumba.ngaphandle kokonakalisa iiseli eziqhelekileyo.Iziphumo zeklinikhi zibonisa ukuba u-Anke Rui unokhuseleko olufanelekileyo kunye nokusebenza kakuhle kwiindidi zamathumba anobungozi.
(4) Glybera
Inkampani: Iphuhliswe yi-uniQure.
Ixesha lokuthengisa: Ivunywe eYurophu ngo-2012.
Izibonakaliso: Unyango lwe-lipoprotein lipase deficiency (LPLD) eneziqephu ezinzima okanye eziphindaphindiweyo ze-pancreatitis nangona ukutya okunamafutha okuthintelwe ngokungqongqo.
Amanqaku: I-Glybera (i-alipogene tiparvovec) liyeza lonyango lofuzo olusekelwe kwi-AAV njenge-vector.Olu nyango lusebenzisa i-AAV njenge-vector yokudlulisa i-LPL ye-gene yonyango kwiiseli ze-muscle, ukwenzela ukuba iiseli ezihambelanayo zikwazi ukuvelisa inani elithile le-lipoprotein lipase, Idlala indima ekupheliseni izifo, kwaye olu nyango lusebenza ixesha elide emva kokulawulwa kwesinye (isiphumo sinokuhlala iminyaka emininzi).Ichiza lacinywa ngo-2017, kwaye izizathu zokukhutshwa kwalo zinokunxulumana nezinto ezimbini: amaxabiso aphezulu kakhulu kunye nemfuno elinganiselweyo yentengiso.I-avareji yeendleko zonyango olunye lwechiza liphezulu ukuya kwi-1 yesigidi seedola zase-US, kwaye sisigulane esinye kuphela esithengileyo saza salisebenzisa ukuza kuthi ga ngoku.Nangona inkampani yeinshorensi yezonyango iyibuyisele nge-900,000 yeedola zaseMelika, ikwangumthwalo omkhulu kwinkampani yeinshurensi.Ukongezelela, ukubonakaliswa kweyeza kunqabile kakhulu, kunye nezinga lezehlo malunga ne-1 kwi-1 yezigidi kunye nezinga eliphezulu lokuxilongwa kakubi.
(5) Imlygic
Inkampani: Iphuhliswe ngu-Amgen.
Ixesha lokuthengisa: Ngo-2015, yamkelwa ukuba ifakwe kuluhlu e-United States kunye ne-European Union.
Iimpawu: Unyango lwezilonda zemelanoma ezingenakususwa ngokupheleleyo ngotyando.
Amagqabantshintshi: Imlygic i genetically modified (ukucima ICP34.5 yayo kunye ICP47 gene amaqhekeza, kwaye ifake igranulocyte-macrophage colony-stimulating factor GM-CSF gene in the virus) attenuated herpes simplex virus type 1 (HSV-1) oncolytic virus, the first FDA-approve virus gened on FDA-approve virus.Indlela yokulawulwa yinaliti ye-intralesional.Isitofu esithe ngqo kwizilonda ze-melanoma sinokubangela ukugqabhuka kweeseli ze-tumor kunye nokukhulula i-antigens ephuma kwi-tumor kunye ne-GM-CSF ukukhuthaza iimpendulo ze-anti-tumor immune.
(6) Luxturna
Inkampani: Iphuhliswe yi-Spark Therapeutics, i-subsidiary ye-Roche.
Ixesha lokuthengisa: Ivunywe yi-FDA kwi-2017, kwaye emva koko ivunyiwe ukuthengisa eYurophu kwi-2018.
Izibonakaliso: Kunyango lwabantwana kunye nabantu abadala abanokulahlekelwa ngumbono ngenxa yokuguquka kwikopi ephindwe kabini ye-RPE65 gene kodwa ngamanani aneleyo eeseli ze-retinal ezisebenzayo.
Amagqabantshintshi: I-Luxturna lunyango lofuzo olusekwe kwi-AAV olulawulwa ngesitofu se-subretinal.Unyango lwe-gene lisebenzisa i-AAV2 njengomphathiswa ukuzisa ikopi esebenzayo ye-RPE65 gene yesiqhelo kwiiseli ze-retinal zesigulane, ukwenzela ukuba iiseli ezihambelanayo ziveze iprotheni ye-RPE65 eqhelekileyo ukuhlawulela isiphene seprotheni ye-RPE65 yesigulane, ngaloo ndlela iphucula umbono wesigulane.
(7) Zolgensma
Inkampani: Iphuhliswe yi-AveXis, i-subsidiary ye-Novartis.
Ixesha lokuthengisa: Ivunywe yi-FDA ngoMeyi ka-2019.
Izibonakaliso: Unyango lwe-spinal muscular atrophy (Spinal Muscular Atrophy, SMA) izigulane ezingaphantsi kweminyaka eyi-2 ubudala.
Amagqabantshintshi: I-Zolgensma lunyango lwemfuza olusekelwe kwi-AAV vector.Eli chiza kuphela kwesicwangciso sonyango lwexesha elinye le-spinal muscular atrophy evunyiweyo ukuba ithengiswe kwihlabathi.iphepha, yinkqubela phambili.Olu nyango lofuzo lusebenzisa i-scAAV9 vector ukwazisa i-SMN1 gene yesiqhelo kwizigulane ngokufaka i-intravenous infusion, ukuvelisa iprotheyini ye-SMN1 eqhelekileyo, ngaloo ndlela iphucula umsebenzi weeseli ezichaphazelekayo ezifana ne-motor neurons.Ngokwahlukileyo koko, iziyobisi ze-SMA i-Spinraza kunye ne-Evrysdi zifuna i-dosing ephindaphindiweyo kwixesha elide, kunye ne-Spinraza elawulwa njengenaliti yomgogodla rhoqo emva kweenyanga ezine, kunye ne-Evrysdi, ichiza lomlomo lemihla ngemihla.
(8) I-Delytact
Inkampani: Iphuhliswe nguDaiichi Sankyo Company Limited (TYO: 4568).
Ixesha lokuthengisa: Imvume enemiqathango evela kuMphathiswa wezeMpilo waseJapan, uMsebenzi kunye neNtlalontle (MHLW) ngoJuni ka-2021.
Iimpawu: Kunyango lwe-glioma enobungozi.
Amagqabantshintshi: I-Delytact yimveliso yesine yonyango lwentsholongwane ye-oncolytic evunyiweyo kwihlabathi liphela kunye nemveliso yokuqala ye-oncolytic yentsholongwane evunyiweyo kunyango lwe-glioma enobungozi.I-Delytact yintsholongwane ye-herpes simplex eyenziwe ngokwemfuza yohlobo loku-1 (HSV-1) oncolytic virus eyenziwe nguGqr Todo kunye noogxa.I-Delytact yazisa utshintsho olongezelelweyo lokucima kwi-G207 genome ye-HSV-1 yesizukulwana sesibini, iphucula ukuphindaphinda kwayo okukhethiweyo kwiiseli zomhlaza kunye nokungeniswa kweempendulo ze-anti-tumor immune, ngelixa igcina iphrofayili ephezulu yokhuseleko.I-Delytact sisizukulwana sesithathu sokuqala se-oncolytic HSV-1 okwangoku kuvavanyo lwezonyango.Ukwamkelwa kweDelytact eJapan kwakusekwe kulingo lwezonyango lwengalo enye yeSigaba sesi-2.Kwizigulane ezine-glioblastoma ephindaphindiweyo, i-Delytact yadibana nesiphelo sokuqala sonyaka omnye wokuphila, kwaye iziphumo zibonise ukuba i-Delytact yenze ngcono kune-G207.Ukuphindaphinda okunamandla kunye nomsebenzi ophezulu we-antitumor.Oku kusebenza kwiimodeli eziqinileyo zethumba kubandakanya ibele, iprostate, schwannoma, nasopharyngeal, hepatocellular, colorectal, malignant peripheral nerve sheath tumors kunye nomhlaza wethyroid.
(9) Upstaza
Inkampani: Iphuhliswe yi-PTC Therapeutics, Inc. (NASDAQ: PTCT).
Ixesha lokuthengisa: Ivunywe yi-EU ngoJulayi 2022.
Isalathiso: Ukusilela kwe-L-amino acid decarboxylase (AADC) enevumba elimnandi, evunyiweyo kunyango lwezigulane ezineenyanga ezili-18 ubudala nangaphezulu.
Amanqakwana: I-Upstaza™ (eladocagene exuparvovec) lunyango lwejini ye-vivo olusebenzisa i-adeno-associated virus type 2 (AAV2) njenge vector.Isigulana sigula ngenxa yokuguquka kwi-gene encoding i-enzyme ye-AADC.I-AAV2 iphethe ijini esempilweni efaka i-enzyme ye-AADC.Isiphumo sonyango sifezekiswa ngendlela yembuyekezo yemfuza.Kwithiyori, idosi enye isebenza ixesha elide.Lunyango lokuqala oluthengisiweyo lwemfuza olufakwe ngqo kwingqondo.Ugunyaziso lokuthengisa lusebenza kuwo onke amazwe angamalungu e-EU angama-27, kunye ne-Iceland, iNorway kunye neLiechtenstein.
(9) Roctavian
Inkampani: Iphuhliswe yi-BioMarin Pharmaceutical (BioMarin).
Ixesha lokuthengisa: Ivunywe yi-EU ngo-Agasti ka-2022.
Iimpawu: Kunyango lwezigulane zabantu abadala abane-hemophilia A enzima ngaphandle kwembali ye-FVIII factor inhibition kunye ne-AAV5 antibody negative.
Amanqaku: I-Roctavian (valoctocogene roxaparvovec) isebenzisa i-AAV5 njenge-vector kwaye isebenzisa umgqugquzeli okhethekileyo wesibindi somntu we-HLP ukuqhuba ukubonakaliswa kwe-human coagulation factor eight (FVIII) kunye ne-B domain isusiwe.Isigqibo seKomishoni yaseYurophu ukuba ivume ukuthengiswa kwe-valoctocogene roxaparvovec isekelwe kwidatha epheleleyo yenkqubo yophuhliso lwezonyango lwechiza.Phakathi kwabo, isigaba III solingo lweklinikhi GENEr8-1 ibonise ukuba xa kuthelekiswa neenkcukacha zonyaka ngaphambi kokubhaliswa, emva kokuba kufakwe enye ye-valoctocogene roxaparvovec, Izifundo zinezinga eliphantsi kakhulu lokuphuma kwegazi ngonyaka (ABR), ukusetyenziswa rhoqo kwe-recombinant factor VIII (F8) amalungiselelo eprotheni, okanye ukwanda okukhulu kwe-F8 umsebenzi wegazi lomzimba.Emva kweeveki ze-4 zonyango, ukusetyenziswa kwezifundo zonyaka ze-F8 kunye ne-ABR ezifuna unyango zancitshiswa nge-99% kunye ne-84%, ngokulandelanayo, umehluko ophawulekayo wezibalo (p <0.001).Iprofayili yokhuseleko yayithandeka, kungekho zifundo ezifumana i-F8 factor inhibition, i-malignancy, okanye i-thrombotic side effects, kwaye akukho ziganeko ezimbi kakhulu ezinxulumene nonyango (SAEs) zichazwe.
3. Iziyobisi ezincinci ze-nucleic acid
(1) Vitravene
Inkampani: Iphuhliswe ngokudibeneyo ngu-Ionis Pharma (owayesakuba ngu-Isis Pharma) kunye noNovartis.
Ixesha lokuthengisa: Ivunywe yi-FDA kunye ne-EU EMA kwi-1998 kunye ne-1999.
Izibonakaliso: Kunyango lwe-cytomegalovirus retinitis kwizigulane ezine-HIV.
Amanqaku: I-Vitravene yi-antisense oligonucleotide ichiza kunye neyeza lokuqala le-oligonucleotide elivunyiweyo lokuthengisa kwihlabathi.Ekuqaleni kweemarike, imfuno yemarike yamachiza e-anti-cytomegalovirus yayingxamiseke kakhulu;ke ngenxa yophuhliso lonyango olusebenzayo lwe-antiretroviral, inani leemeko ze-cytomegalovirus lehla kakhulu.Ngenxa yemfuno ephantsi yemarike, ichiza lakhululwa ngo-2002 kunye no-2006 Ukurhoxiswa kumazwe e-EU nase-US.
(2) Macugen
Inkampani: Iphuhliswe ngokudibeneyo yiPfizer kunye ne-Eyetech.
Ixesha lokuthengisa: Ivunyiwe ukuba ifakwe e-United States ngo-2004.
Iimpawu: Kunyango lwe-neovascular age-related macular degeneration.
Amanqaku: I-Macugen yi-pegylated modified oligonucleotide drug enokuthi ijolise kwaye ibophe kwi-vascular endothelial growth factor (VEGF165 isoform), kwaye ilawulwa nge-injection ye-intravitreal.
(3) Defitelio
Inkampani: Iphuhliswe yiJazz.
Ixesha lokuthengisa: Ivunywe yi-European Union kwi-2013, kwaye ivunywe yi-FDA ngo-Matshi 2016.
Iimpawu: Kunyango lwe-hepatic venule occlusive disease ehambelana ne-renal okanye i-pulmonary dysfunction emva kwe-hematopoietic stem cell transplantation.
Amanqaku: I-Defitelio yiziyobisi ze-oligonucleotide, umxube we-oligonucleotides kunye neempawu ze-plasmin.Yarhoxiswa ngo-2009 ngenxa yezizathu zorhwebo.
(4) Kynamro
Inkampani: Iphuhliswe ngokudibeneyo ngu-Ionis Pharma kunye neKastle.
Ixesha lokuthengisa: Ivunywe eUnited States njengeyeza lentandane ngo-2013.
Iimpawu: Kunyango lwe-adjuvant ye-homozygous yosapho lwe-hypercholesterolemia.
Ukuphawula: I-Kynamro yi-antisense oligonucleotide drug, i-oligonucleotide ye-antisense ejolise kwi-apo yabantu B-100 mRNA.I-Kynamro ilawulwa njenge-200 mg ngaphantsi kwesikhumba kanye ngeveki.
(5) Spinraza
Inkampani: Iphuhliswe yi-Ionis Pharmaceuticals.
Ixesha lokuthengisa: Ivunywe yi-FDA ngoDisemba 2016.
Izibonakaliso: Kunyango lwe-spinal muscular atrophy (SMA).
Amagqabantshintshi: I-Spinraza (nusinersen) sisiyobisi esichasene ne-oligonucleotide.I-Spinraza inokutshintsha i-RNA ye-splicing ye-SMN2 gene ngokuzibophelela kwindawo ye-splicing ye-SMN2 exon 7, ngaloo ndlela ikhulise ukuveliswa kweprotheyini ye-SMN esebenza ngokupheleleyo.Ngo-Agasti 2016, i-BIOGEN Corporation isebenzise ukhetho lwayo lokufumana amalungelo ehlabathi e-Spinraza.I-Spinraza yaqala ulingo lwayo lokuqala lweklinikhi kubantu kwi-2011. Kwiminyaka eyi-5 nje, yavunywa yi-FDA kwi-2016, ebonisa ukuqaphela ngokupheleleyo kwe-FDA ukusebenza kwayo.Ichiza livunyiwe ukuba lithengiswe e-China ngo-Ephreli 2019. Umjikelezo wonke wokuvunywa kwe-Spinraza e-China ungaphantsi kweenyanga ze-6.Sekuyiminyaka eyi-2 kunye neenyanga ze-2 ukususela ekubeni i-Spinraza yamkelwa okokuqala e-United States.I-blockbuster enjalo isifo esinqabileyo sangaphandle iyeza elitsha liku- Isantya soluhlu e-China sele sikhawuleza kakhulu.Oku kukwabangelwa "Isaziso soKhupho loLuhlu lokuQala lwe-Overseas New Drugs olufunekayo ngokukhawuleza kuPhando lweKlinikhi" olukhutshwe yiZiko loVavanyo lweziyobisi ngoNovemba 1, 2018, elifakwe kwibhetshi yokuqala ye-40 engundoqo amachiza amatsha angaphandle ukuphononongwa ngokukhawuleza, kunye ne-Spinraza ibekwe kwindawo.
(6) IiEksodus 51
Inkampani: Iphuhliswe yi-AVI BioPharma (kamva yathiywa ngokuba yiSarepta Therapeutics).
Ixesha lokuthengisa: Ivunywe yi-FDA ngoSeptemba 2016.
Iimpawu: Kunyango lwe-Duchenne muscular dystrophy (DMD) kunye nokuguqulwa kofuzo lwe-DMD kwi-exon 51 ye-skipping gene.
Amagqabantshintshi: I-Exondys 51 yi-antisense oligonucleotide ichiza.I-oligonucleotide ye-antisense inokubophelela kwi-exon 51 isikhundla se-pre-mRNA ye-DMD gene, okubangelwa ukubunjwa kwe-mRNA evuthiweyo.I-Excision, ngaloo ndlela ilungisa ngokuyinxenye isakhelo sokufunda se-mRNA, inceda isigulane ukuba sidibanise ezinye iindlela zokusebenza ze-dystrophin ezimfutshane kuneprotheni eqhelekileyo, ngaloo ndlela kuphuculwe iimpawu zesigulana.
(7) Tegsedi
Inkampani: Iphuhliswe yi-Ionis Pharmaceuticals.
Ixesha lokuthengisa: Ivunywe yiManyano yaseYurophu ukuba ithengiswe ngoJulayi ka-2018.
Iimpawu: Kunyango lwe-hereditary transthyretin amyloidosis (hATTR).
Amagqabantshintshi: I-Tegsedi lichiza le-antisense oligonucleotide elijolise kwi-transthyretin mRNA.Lichiza lokuqala elivunyiweyo kwihlabathi kunyango lwe-hATTR.Indlela yokulawula i-injection subcutaneous.Ichiza linciphisa ukuveliswa kweprotheyini ye-ATTR ngokujolisa kwi-mRNA ye-transthyretin (ATTR), kwaye inomlinganiselo omhle wenzuzo-umngcipheko kunyango lwe-ATTR.Akukho nqanaba lesifo okanye ubukho be-cardiomyopathy obufanelekileyo.
(8) Onpattro
Inkampani: Iphuhliswe ngokubambisana yi-Alnylam kunye ne-Sanofi.
Ixesha lokuthengisa: Ivunyiwe ukuba ifakwe e-United States ngo-2018.
Iimpawu: Kunyango lwe-hereditary transthyretin amyloidosis (hATTR).
Amanqaku: I-Onpattro yi-siRNA ichiza elijolise kwi-transthyretin mRNA, eyanciphisa ukuveliswa kweprotheyini ye-ATTR kwisibindi kunye nokuqokelela i-amyloid deposits kwi-peripheral nerves ngokujolisa kwi-mRNA ye-transthyretin (ATTR)., ngaloo ndlela kuphuculwe kwaye kuthomalaliswe iimpawu zesifo.
(9) Givlaari
Inkampani: Iphuhliswe yi-Alnylam Corporation.
Ixesha lokuthengisa: Ivunywe yi-FDA ngoNovemba ka-2019.
Izibonakaliso: Kunyango lwe-acute hepatic porphyria (AHP) kubantu abadala.
Amagqabantshintshi: I-Givlaari lichiza le-siRNA, ichiza lesibini le-siRNA elivunyiweyo ukuthengiswa emva kwe-Onpattro.Ichiza lilawulwa ngaphantsi kwesikhumba kwaye lijolise kwi-mRNA yokuthotywa kweprotheni ye-ALAS1.Unyango lwenyanga lwe-Givlaari lunokunciphisa kakhulu kwaye ngokuqhubekayo inqanaba le-ALAS1 esibindini, ngaloo ndlela linciphisa amanqanaba e-neurotoxic ALA kunye ne-PBG kuluhlu oluqhelekileyo, ngaloo ndlela Nciphisa iimpawu zesifo sesigulane.Idatha ibonise ukuba izigulane eziphathwe nge-Givlaari zine-74% yokunciphisa inani lezifo zesifo xa kuthelekiswa neqela le-placebo.
(10) IiVyondys53
Inkampani: Iphuhliswe yiSarepta Therapeutics.
Ixesha lokuthengisa: Ivunywe yi-FDA ngoDisemba ka-2019.
Isalathisi: Kunyango lwezigulane ze-DMD ezine-dystrophin gene exon 53 splice mutation.
Amagqabantshintshi: I-Vyondys 53 lichiza elichasene ne-oligonucleotide.Ichiza le-oligonucleotide lijolise kwinkqubo ye-splicing ye-dystrophin mRNA precursor.Kwinkqubo engathanga ngqo ye-dystrophin mRNA precursor, i-Exon 53 yangaphandle yahlulwe ngokuyinxenye, okt ayikho kwi-mRNA ekhulileyo, kwaye yenzelwe ukuvelisa iprotheni ye-dystrophin ecutshiweyo kodwa esebenzayo, ngaloo ndlela iphucula umthamo wokuzilolonga kwizigulana.
(11) Waylivra
Inkampani: Iphuhliswe yi-Ionis Pharmaceuticals kunye ne-Akcea Therapeutics encedisayo.
Ixesha lokuthengisa: Ivunywe yi-Arhente yaMayeza yaseYurophu (EMA) ngoMeyi ka-2019.
Isalathisi: Njengonyango oluncedisayo kwisondlo esilawulwayo kwizigulane zabantu abadala abane-familial chylomicronemia syndrome (FCS).
Amagqabantshintshi: I-Waylivra lichiza le-antisense oligonucleotide, elichiza lokuqala elivunyiweyo kunyango lwe-FCS kwihlabathi.
(12) Leqvio
Inkampani: Iphuhliswe nguNovartis.
Ixesha lokuthengisa: Ivunywe yi-EU ngoDisemba ka-2020.
Izibonakaliso: Kunyango lwe-hypercholesterolemia yabantu abadala (i-heterozygous yentsapho kunye ne-non-familial) okanye i-dyslipidemia edibeneyo.
Amagqabantshintshi: I-Leqvio lichiza le-siRNA elijolise kwi-PCSK9 mRNA.Lunyango lokuqala lwehlabathi lokwehlisa i-cholesterol (i-LDL-C) lwe-siRNA.Indlela yokulawula i-injection subcutaneous.Ichiza lisebenza ngokuphazamiseka kwe-RNA ekunciphiseni amanqanaba eprotheyini ye-PCSK9, leyo iyancipha amanqanaba e-LDL-C.Idatha yezonyango ibonisa ukuba iLeqvio inokwehlisa i-LDL-C malunga ne-50% kwizigulana ezinamaqondo e-LDL-C angenako ukuthotywa ukuya kumanqanaba ekujoliswe kuwo nangona idosi ephezulu enyanyezelweyo yeestatins.
(13) Uxolo
Inkampani: Iphuhliswe yi-Alnylam Pharmaceuticals.
Ixesha lokuthengisa: Ivunywe yi-EU ngoNovemba ka-2020.
Izibonakaliso: Kunyango lwe-hyperoxaluria yokuqala yohlobo lwe-1 (PH1).
Amanqaku: I-Oxlumo lichiza le-siRNA elijolise kwi-hydroxy acid oxidase 1 (HAO1) mRNA, elawulwa ngaphantsi kwe-subcutaneously.Ichiza laphuhliswa kusetyenziswa i-Alnylam yekhemikhali yamva nje yokuqinisa uzinzo ye-ESC-GalNAc itekhnoloji yokudibanisa, eyenza ukuba i-siRNAs elawulwa ngaphantsi kwesikhumba isebenze ngokuzingisa okukhulu kunye nokusebenza kakuhle.Ichiza lijolise ekuthotyweni okanye ekuvinjweni kwe-hydroxy acid oxidase 1 (HAO1) mRNA, inciphisa inqanaba le-glycolate oxidase esibindini, emva koko idle i-substrate efunekayo kwimveliso ye-oxalate kwaye inciphisa ukuveliswa kwe-oxalate ukulawula ukuqhubela phambili kwesifo kunye nokuphucula iimpawu zesifo kwizigulane.
(14) Viltepso
Inkampani: Iphuhliswe ngu-NS Pharma, i-subsidiary ye-Nippon Shinyaku.
Ixesha lokuthengisa: Ivunywe yi-FDA ngo-Agasti ka-2020.
Iimpawu: Kunyango lwe-Duchenne muscular dystrophy (DMD) kunye nokuguqulwa kofuzo lwe-DMD kwi-exon 53 ye-skipping gene.
Amagqabantshintshi: IViltepso lichiza le-phosphorodiamide morpholino oligonucleotide.Eli chiza le-oligonucleotide linokubophelela kwi-exon 53 isikhundla se-pre-mRNA ye-DMD gene, okubangelwa ukubunjwa kwe-mRNA ekhulile.I-exon isuswe ngokuyinxenye, ngaloo ndlela ilungisa ngokuyinxenye isakhelo sokufunda se-mRNA, inceda isigulane ukuba sidibanise ezinye iindlela zokusebenza ze-dystrophin ezimfutshane kuneprotheni eqhelekileyo, ngaloo ndlela kuphuculwe iimpawu zesigulana.
(15) Amvuttra (vutrisiran)
Inkampani: Iphuhliswe yi-Alnylam Pharmaceuticals.
Ixesha lokuthengisa: Ivunywe yi-FDA ngoJuni 2022.
Iimpawu: Kunyango lwe-transthyretin amyloidosis yelifa lomntu omdala kunye ne-polyneuropathy (hATTR-PN).
Amanqakwana: I-Amvuttra (Vutrisiran) lichiza le-siRNA elijolise kwi-transthyretin (ATTR) mRNA, elawulwa ngesitofu esingaphantsi kwesikhumba.I-Vutrisiran yenzelwe ngokusekelwe kwi-Alnylam's Enhanced Stabilization Chemistry (ESC)-GalNAc iqonga elidityanisiweyo lonikezelo kunye nokwanda kwamandla kunye nokuzinza kwe-metabolic.Ukuvunywa kwonyango kusekelwe kwidatha yeenyanga ze-9 ukusuka kwiSigaba sesi-III sophando lweklinikhi (HELIOS-A), kunye neziphumo ezipheleleyo ezibonisa ukuba unyango luphucule iimpawu ze-hATTR-PN, ngaphezu kwe-50% yezigulane ezibuyisela umva okanye ukuyeka ukuqhubeka.
4. Amanye amayeza onyango lwemfuza
(1) Rexin-G
Inkampani: Iphuhliswe yi-Epeius Biotech.
Ixesha lokuthengisa: Ivunywe yiPhilippine Food and Drug Administration (BFAD) kwi-2005.
Izalathisi: Kunyango lwemihlaza ephezulu ekwaziyo ukumelana nechemotherapy.
Amagqabantshintshi: I-Rexin-G yinaliti ye-nanoparticle enemfuza.Yazisa i-cyclin G1 yejini eguqukayo kwiiseli ekujoliswe kuzo ngokusebenzisa i-retroviral vector ukubulala ngokukodwa amathumba aqinileyo.Indlela yokulawula i-intravenous infusion.Njengechiza elijoliswe kwithumba elifuna ngenkuthalo kwaye litshabalalise iiseli zomhlaza we-metastatic, linefuthe elithile kwizigulana ezingasebenziyo ngokuchasene namanye amachiza omhlaza, kubandakanywa ibhayoloji ekujoliswe kuyo.
(2) Neovasculgen
Inkampani: Iphuhliswe liziko le-Human stem cells.
Ixesha lokudweliswa: Livunyiwe ukuba lifakwe eRashiya ngoDisemba 7, 2011, laza ladweliswa eUkraine ngo-2013.
Izibonakaliso: Kunyango lwe-peripheral arterial disease, kubandakanywa ne-ischemia yelungu elibi.
Amanqaku: I-Neovasculgen yi-DNA plasmid-based based gene therapy apho i-vascular endothelial growth factor (VEGF) i-165 gene yakhiwe kwi-backbone ye-plasmid kwaye ifakwe kwizigulane.
(3) Collategene
Inkampani: Iphuhliswe ngokudibeneyo yiYunivesithi yaseOsaka kunye neefemu zenkunzi yemali.
Ixesha lokudwelisa: Ivunywe nguMphathiswa wezeMpilo waseJapan, uMsebenzi kunye neNtlalontle ukuba ifakwe kuluhlu ngo-Agasti ka-2019.
Izibonakaliso: Unyango lwe-ischemia ephantsi kakhulu.
Amagqabantshintshi: I-Collategene lunyango lwemfuza olusekwe kwi-plasmid, iyeza lokuqala lomthonyama laseJapan lonyango lwemfuza eliveliswe yi-AnGes.Inxalenye ephambili yeli chiza yi-plasmid ehamba ze equlethe ulandelelwano lwemfuza ye-human hepatocyte factor factor (HGF).Ukuba ichiza litofelwe kwizihlunu zamalungu asezantsi, i-HGF echaziweyo iya kukhuthaza ukwakheka kwemithambo yegazi emitsha ejikeleze imithambo yegazi evalekileyo.Ulingo lwezonyango luqinisekisile ukusebenza kwayo ekuphuculeni izilonda.
ISIPHELO
Ixesha lokuposa: Nov-10-2022